Yonsei Med J.
2019 Sep;60(9):854-863. 10.3349/ymj.2019.60.9.854.
Effect of Relaxin Expression from an Alginate Gel-Encapsulated Adenovirus on Scar Remodeling in a Pig Model
- Affiliations
-
- 1Institute for Human Tissue Restoration, Department of Plastic & Reconstructive Surgery, Yonsei University College of Medicine, Seoul, Korea. pswjlee@yuhs.ac
- 2Department of Bioengineering, College of Engineering, Hanyang University, Seoul, Korea. chaeok@hanyang.ac.kr
- 3Institute of Nano Science and Technology (INST), Hanyang University, Seoul, Korea.
- 4GeneMedicine Co., Ltd., Seoul, Korea.
- KMID: 2457481
- DOI: http://doi.org/10.3349/ymj.2019.60.9.854
Abstract
- PURPOSE
Relaxin (RLX) is a transforming growth factor-β1 (TGF-β1) antagonist that is believed to function as a potent collagen re-arranger and a major suppressor of extracellular matrix components. Adenoviruses (Ads) are accepted vectors for cancer gene therapy. However, repeated treatments of Ad are limited by short-term biological activity in vivo. The efficacy of sustained RLX expression to scar remodeling was assessed using an injectable alginate gel-matrix system.
MATERIALS AND METHODS
Pig scar tissue was treated with relaxin-expressing Ad loaded in alginate gel (gel/Ad-RLX). Surface areas, color, and pliability of scars were compared, and various factors influencing scar formation and collagen arrangement were analyzed.
RESULTS
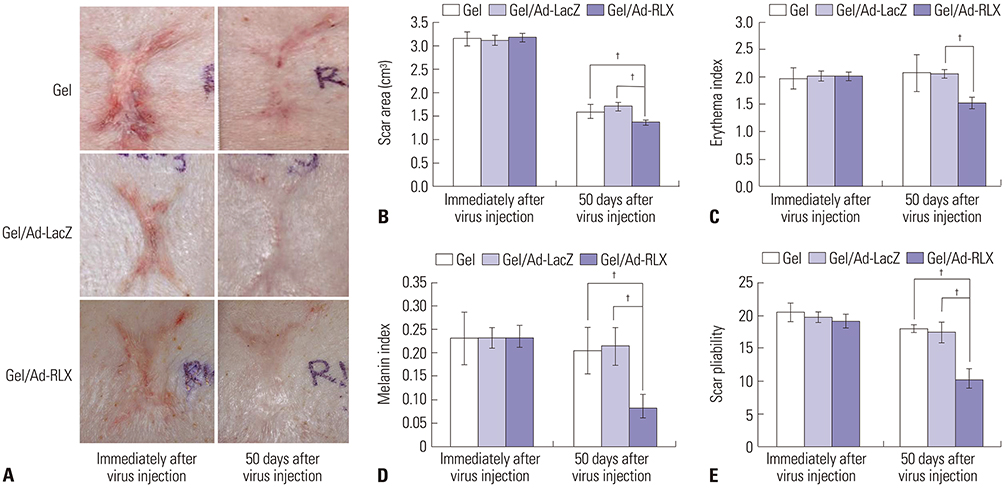
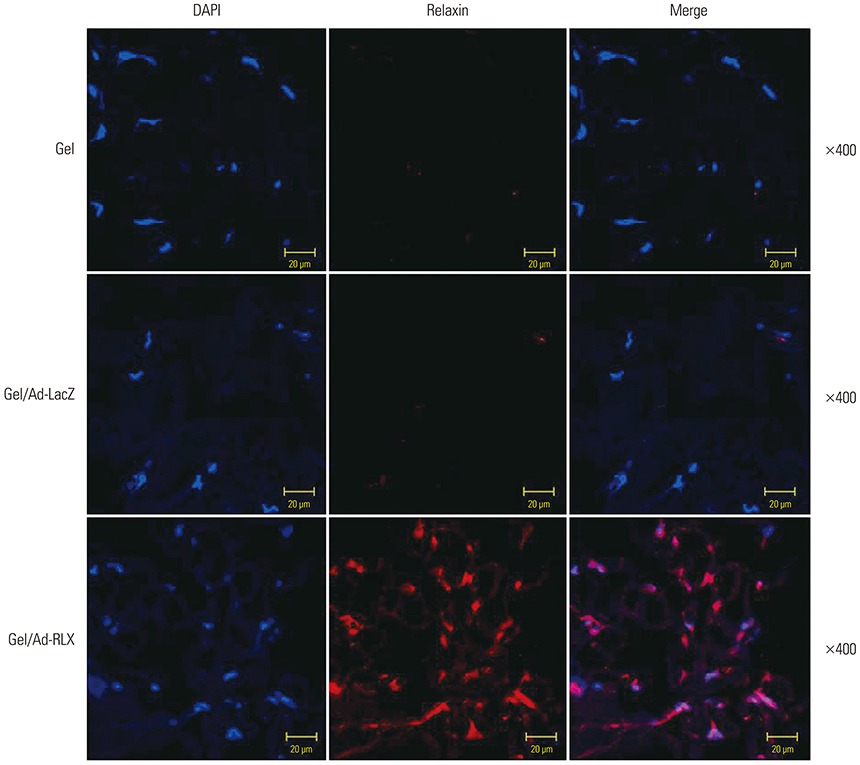
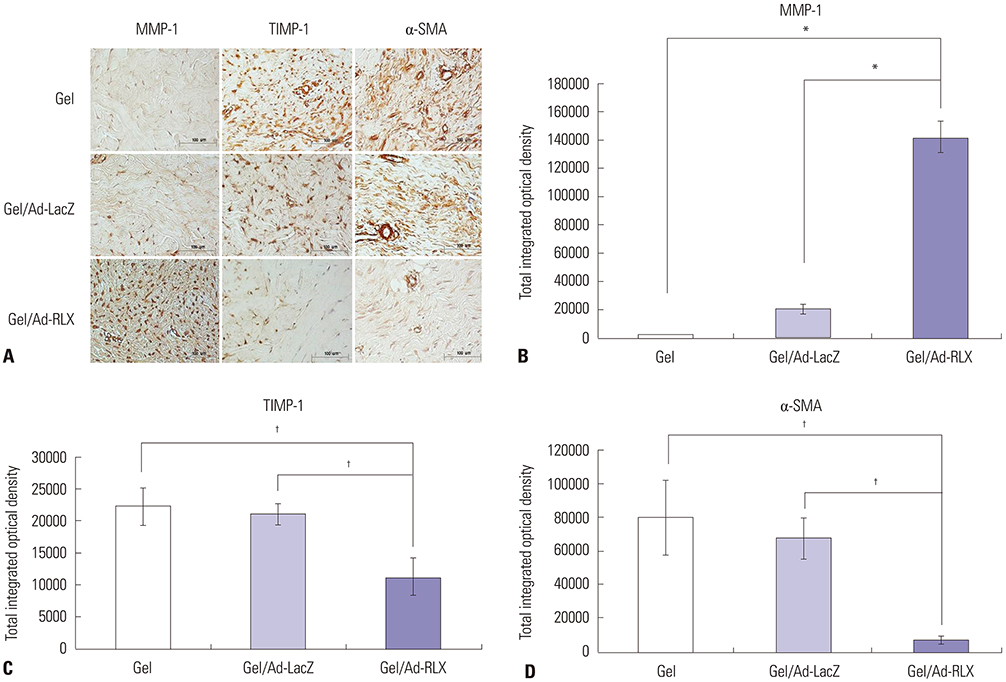
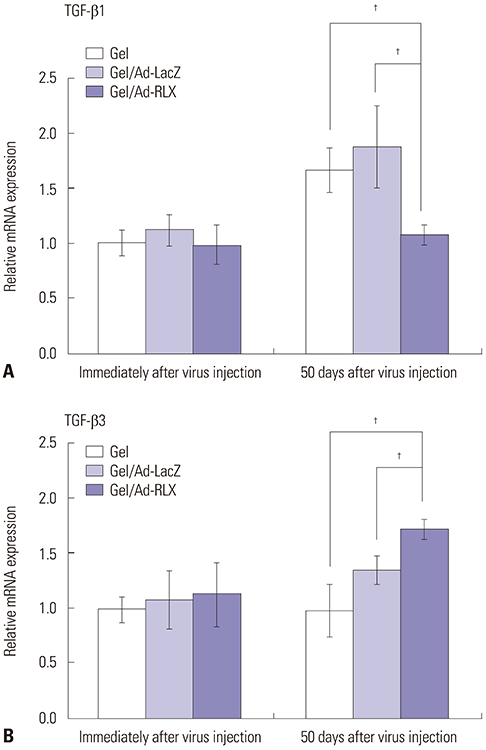
Gel/Ad-RLX decreased scar size, color index, and pliability. Immunohistochemistry showed decreased levels of major extracellular matrix proteins in the gel/Ad-RLX-treated group. Furthermore, treatment with gel/Ad-RLX reduced expression of tissue inhibitor of metalloproteinase-1 and alpha-smooth muscle actin and markedly increased expression of matrix metalloproteinase-1 in pig scar tissues. Gel/Ad-RLX also significantly downregulated TGF-β1 and upregulated TGF-β3 mRNAs in pig scar tissues.
CONCLUSION
These results support a prominent role for RLX in scar remodeling and suggest that gel/Ad-RLX may have therapeutic effects on scar formation.
Keyword
MeSH Terms
-
Actins
Adenoviridae*
Cicatrix*
Collagen
Extracellular Matrix
Extracellular Matrix Proteins
Genes, Neoplasm
Genetic Therapy
Immunohistochemistry
Matrix Metalloproteinase 1
Pliability
Relaxin*
RNA, Messenger
Therapeutic Uses
Tissue Inhibitor of Metalloproteinase-1
Actins
Collagen
Extracellular Matrix Proteins
Matrix Metalloproteinase 1
RNA, Messenger
Relaxin
Therapeutic Uses
Tissue Inhibitor of Metalloproteinase-1
Figure
Reference
-
1. Desmoulière A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003; 83:1689–1707.
Article2. Le AD, Brown JJ. Wound healing: repair biology and wound and scar treatment. In : Bagheri SC, Bell RB, Khan HA, editors. Current therapy in oral and maxillofacial surgery. 1st ed. Philadelphia: Saunders;2012. p. 6–10.3. Bae SH, Bae YC. Analysis of frequency of use of different scar assessment scales based on the scar condition and treatment method. Arch Plast Surg. 2014; 41:111–115.
Article4. Corr DT, Gallant-Behm CL, Shrive NG, Hart DA. Biomechanical behavior of scar tissue and uninjured skin in a porcine model. Wound Repair Regen. 2009; 17:250–259.
Article5. Wang XQ, Kravchuk O, Liu PY, Kempf M, Boogaard CV, Lau P, et al. The evaluation of a clinical scar scale for porcine burn scars. Burns. 2009; 35:538–546.
Article6. Wang XQ, Liu PY, Kempf M, Cuttle L, Chang AH, Wong M, et al. Burn healing is dependent on burn site: a quantitative analysis from a porcine burn model. Burns. 2009; 35:264–269.
Article7. Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK, Tark KC, et al. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: a pilot study. Dermatol Surg. 2012; 38:1678–1688.
Article8. Zhao S, Lee HY, Sherwood OD. Porcine and human relaxin bioactivity: bioactivities of porcine relaxin and human relaxin do not differ in mice and rats. Ann N Y Acad Sci. 2005; 1041:126–131.
Article9. Samuel CS, Hewitson TD, Unemori EN, Tang ML. Drugs of the future: the hormone relaxin. Cell Mol Life Sci. 2007; 64:1539–1557.
Article10. Sherwood OD. Relaxin's physiological roles and other diverse actions. Endocr Rev. 2004; 25:205–234.
Article11. Samuel CS, Hewitson TD. Relaxin in cardiovascular and renal disease. Kidney Int. 2006; 69:1498–1502.
Article12. Dschietzig T, Bartsch C, Baumann G, Stangl K. Relaxin-a pleiotropic hormone and its emerging role for experimental and clinical therapeutics. Pharmacol Ther. 2006; 112:38–56.
Article13. Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, et al. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod. 1999; 14:800–806.
Article14. Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006; 98:1482–1493.
Article15. Unemori EN, Lewis M, Constant J, Arnold G, Grove BH, Normand J, et al. Relaxin induces vascular endothelial growth factor expression and angiogenesis selectively at wound sites. Wound Repair Regen. 2000; 8:361–370.
Article16. Chartier C, Degryse E, Gantzer M, Dieterle A, Pavirani A, Mehtali M. Efficient generation of recombinant adenovirus vectors by homologous recombination in Escherichia coli. J Virol. 1996; 70:4805–4810.
Article17. Kim J, Cho JY, Kim JH, Jung KC, Yun CO. Evaluation of E1B gene-attenuated replicating adenoviruses for cancer gene therapy. Cancer Gene Ther. 2002; 9:725–736.
Article18. Lee WJ, Choi IK, Lee JH, Lee JS, Kim YO, Rah DK, et al. Relaxin-expressing adenovirus decreases collagen synthesis and up-regulates matrix metalloproteinase expression in keloid fibroblasts: in vitro experiments. Plast Reconstr Surg. 2012; 130:407e–417e.19. Lee WJ, Kim YO, Choi IK, Rah DK, Yun CO. Adenovirus-relaxin gene therapy for keloids: implication for reversing pathological fibrosis. Br J Dermatol. 2011; 165:673–677.
Article20. Lee WJ, Yun CO, Yun IS, Kim YO, Choi IK, Yun TJ, et al. Augmentation of rat skin flap viability by relaxin-expressing adenovirus. Wound Repair Regen. 2011; 19:709–717.
Article21. Ahi YS, Bangari DS, Mittal SK. Adenoviral vector immunity: its implications and circumvention strategies. Curr Gene Ther. 2011; 11:307–320.
Article22. Choi JW, Kang E, Kwon OJ, Yun TJ, Park HK, Kim PH, et al. Local sustained delivery of oncolytic adenovirus with injectable alginate gel for cancer virotherapy. Gene Ther. 2013; 20:880–892.
Article23. Park H, Kim PH, Hwang T, Kwon OJ, Park TJ, Choi SW, et al. Fabrication of cross-linked alginate beads using electrospraying for adenovirus delivery. Int J Pharm. 2012; 427:417–425.
Article24. Choi KJ, Zhang SN, Choi IK, Kim JS, Yun CO. Strengthening of antitumor immune memory and prevention of thymic atrophy mediated by adenovirus expressing IL-12 and GM-CSF. Gene Ther. 2012; 19:711–723.
Article25. Zhang SN, Choi IK, Huang JH, Yoo JY, Choi KJ, Yun CO. Optimizing DC vaccination by combination with oncolytic adenovirus coexpressing IL-12 and GM-CSF. Mol Ther. 2011; 19:1558–1568.
Article26. Yun IS, Lee WJ, Rah DK, Kim YO, Park BY. Skin color analysis using a spectrophotometer in Asians. Skin Res Technol. 2010; 16:311–315.
Article27. Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am. 2006; 26:387–405.
Article28. Sur R, Cavender D, Malaviya R. Different approaches to study mast cell functions. Int Immunopharmacol. 2007; 7:555–567.
Article29. Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009; 228:149–169.
Article30. Park BY, Shin IS, Yun IS. Dovetail scar revision. Dermatol Surg. 2012; 38:1716–1721.
Article31. Kim SG, Kim EY, Kim YJ, Lee SI. The efficacy and safety of ablative fractional resurfacing using a 2,940-Nm Er:YAG laser for traumatic scars in the early posttraumatic period. Arch Plast Surg. 2012; 39:232–237.
Article32. Greenhalgh DG. Consequences of excessive scar formation: dealing with the problem and aiming for the future. Wound Repair Regen. 2007; 15:Suppl 1. S2–S5.
Article33. Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995; 108(Pt 3):985–1002.
Article34. Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003; 11:25–34.
Article35. Liu W, Chua C, Wu X, Wang D, Ying D, Cui L, et al. Inhibiting scar formation in rat wounds by adenovirus-mediated overexpression of truncated TGF-beta receptor II. Plast Reconstr Surg. 2005; 115:860–870.
Article36. Gallant CL, Olson ME, Hart DA. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen. 2004; 12:305–319.
Article37. Margulis A, Nocka KH, Wood NL, Wolf SF, Goldman SJ, Kasaian MT. MMP dependence of fibroblast contraction and collagen production induced by human mast cell activation in a three-dimensional collagen lattice. Am J Physiol Lung Cell Mol Physiol. 2009; 296:L236–L247.
Article38. Samuel CS, Cendrawan S, Gao XM, Ming Z, Zhao C, Kiriazis H, et al. Relaxin remodels fibrotic healing following myocardial infarction. Lab Invest. 2011; 91:675–690.
Article39. Stewart DR. Scar prevention and cosmetically enhanced wound healing using relaxin. Ann N Y Acad Sci. 2009; 1160:336–341.
Article40. Du XJ, Xu Q, Lekgabe E, Gao XM, Kiriazis H, Moore XL, et al. Reversal of cardiac fibrosis and related dysfunction by relaxin. Ann N Y Acad Sci. 2009; 1160:278–284.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Relaxin Expressing Adenovirus on Scar Remodeling: A Preliminary Study
- Effect of Thermoresponsive Poly(L-lactic acid)-poly (ethylene glycol) Gel Injection on Left Ventricular Remodeling in a Rat Myocardial Infarction Model
- Preadipocyte Culture in Chitosan-Alginate Gel
- Relaxin Modulates the Expression of MMPs and TIMPs in Fibroblasts of Patients with Carpal Tunnel Syndrome
- The Effect of Poly-L-lysine on Proliferation and Differentiation of Preadipocyte in the Alginate Gels