Cancer Res Treat.
2016 Apr;48(2):715-726. 10.4143/crt.2015.227.
Caveolin-1 Modulates Docetaxel-Induced Cell Death in Breast Cancer Cell Subtypes through Different Mechanisms
- Affiliations
-
- 1Division of Oncology/Hematology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. khpark@korea.ac.kr
- 2Division of Radiation Cancer Biology, Korea Institute of Radiological & Medical Sciences, Seoul, Korea.
- 3Department of Pathology, Korea University College of Medicine, Seoul, Korea.
- KMID: 2454350
- DOI: http://doi.org/10.4143/crt.2015.227
Abstract
- PURPOSE
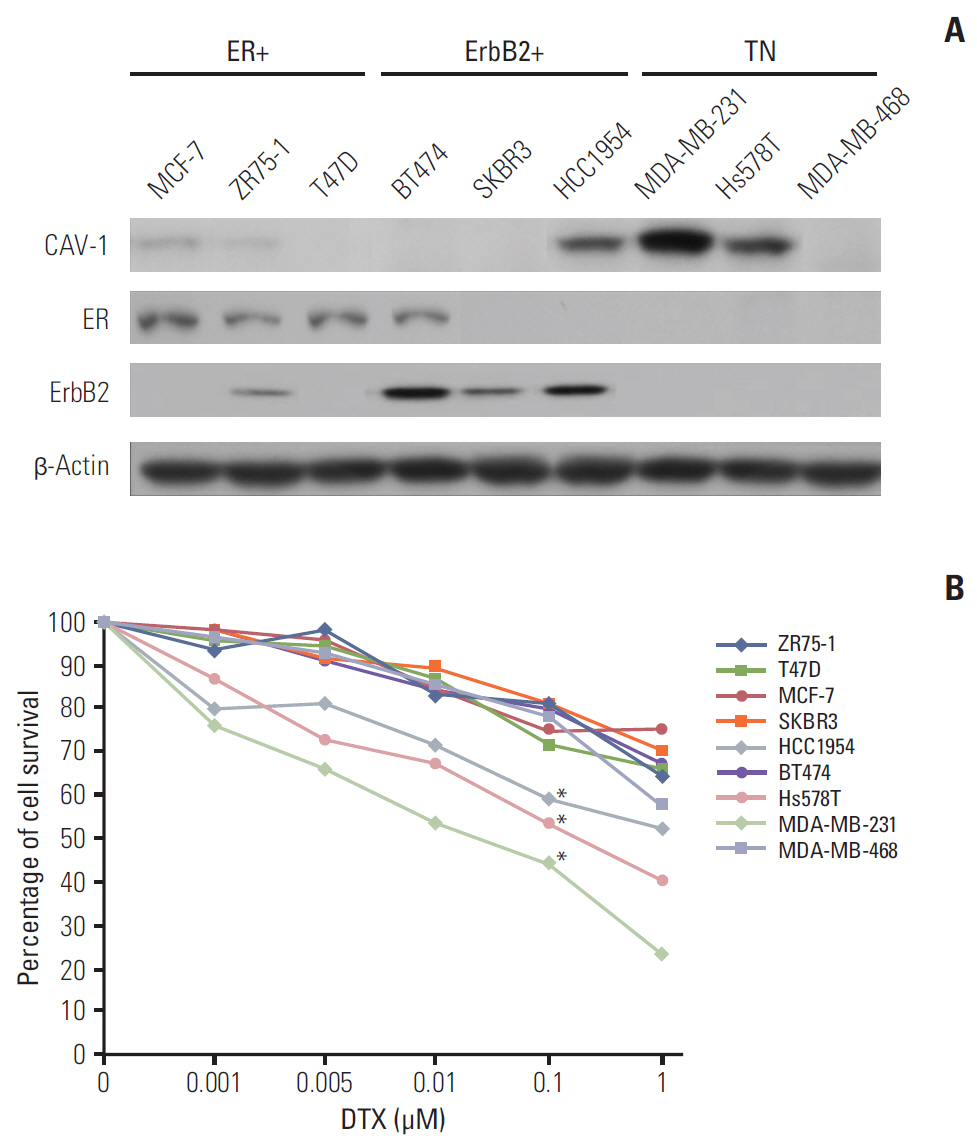
Caveolin-1 (CAV-1) expression is more associated with basal-like cancers than estrogen receptor- or ErbB-2-expressing breast cancers. However, the biological relevance of different levels of CAV-1 expression according to subtype in the epithelial compartment of breast cancer remains unclear.
MATERIALS AND METHODS
We investigated whether CAV-1 functions as a tumor suppressor and/or modulator of the cytotoxic activity of docetaxel (DTX) in subtypes of breast cancer using in vitro and xenograft models.
RESULTS
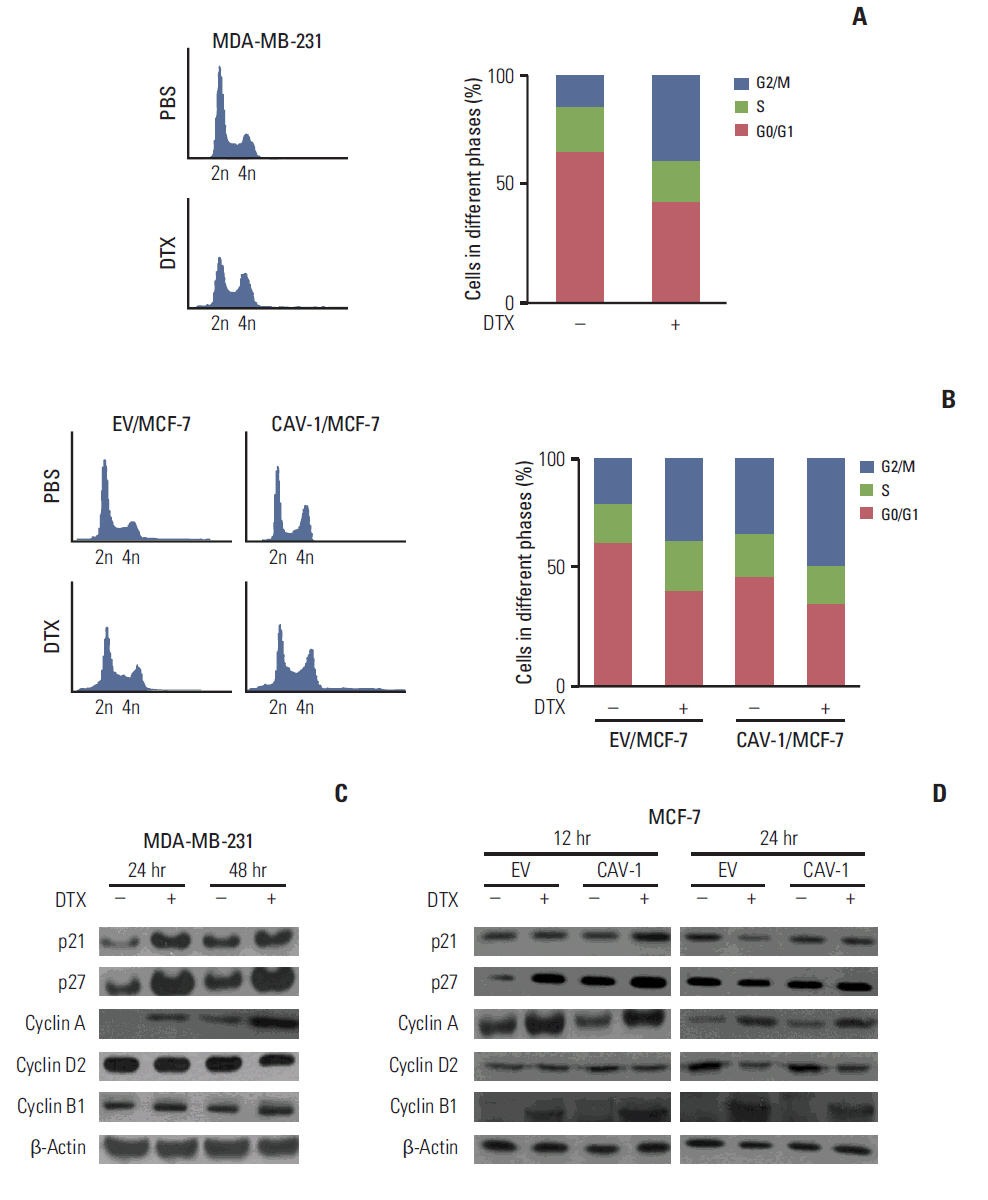
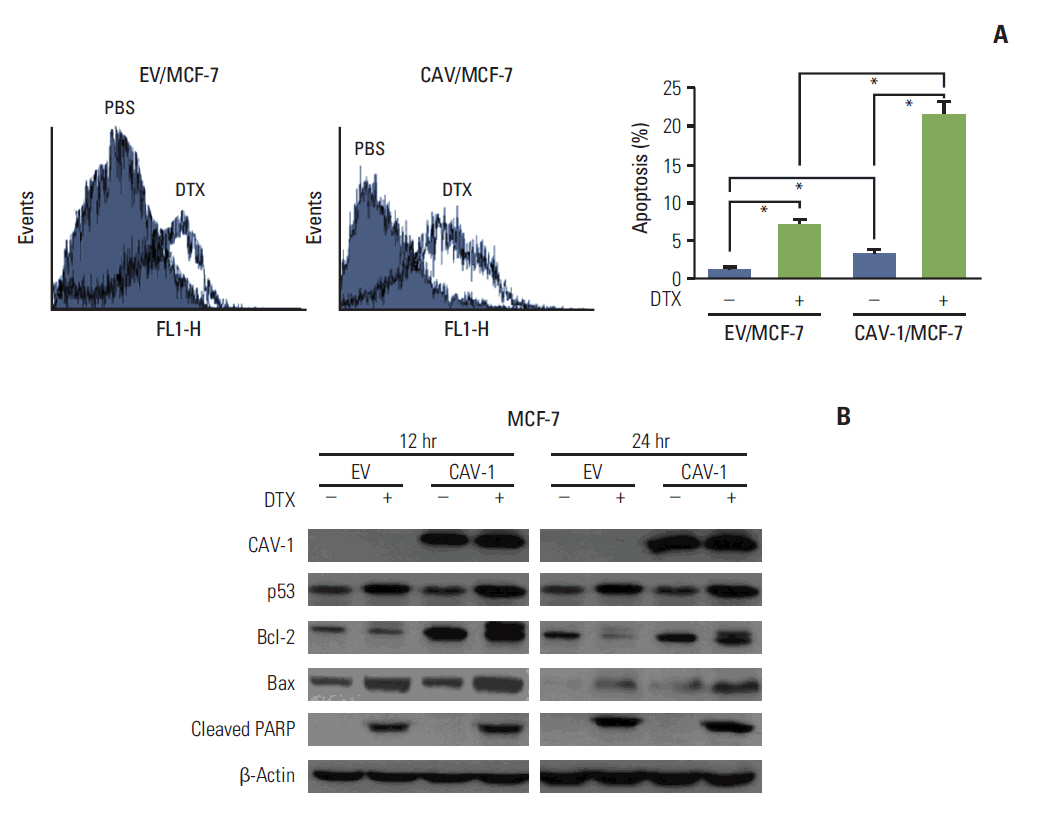
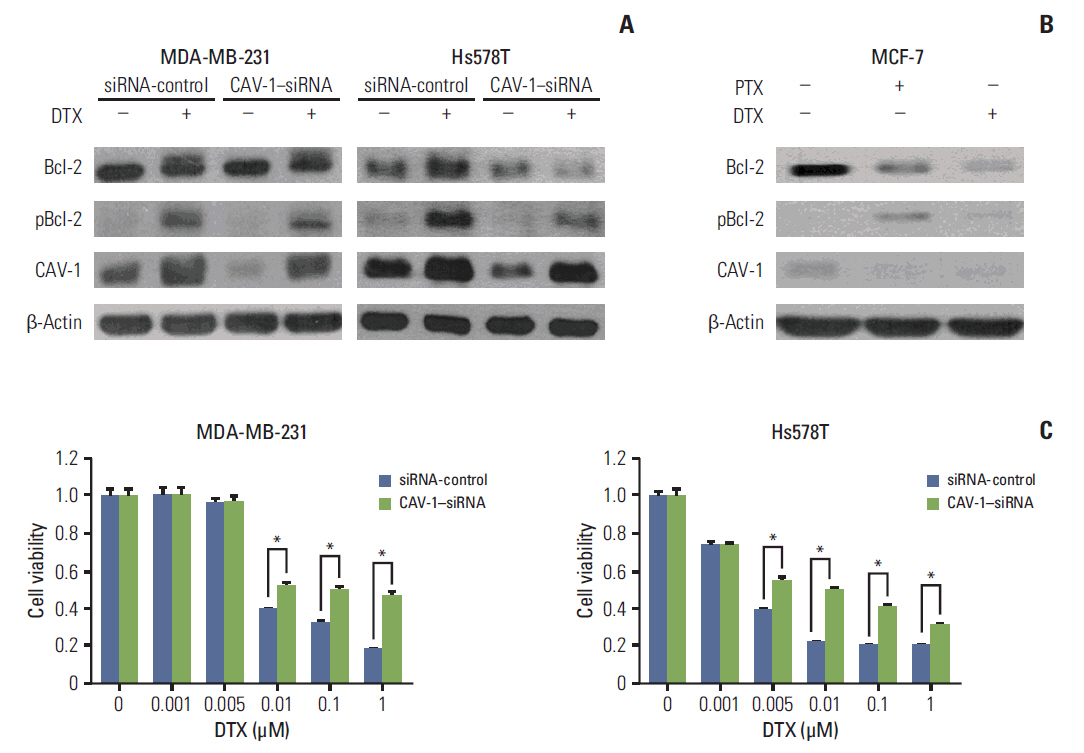
The levels of CAV-1 expression were closely associated with DTX sensitivity in triple-negative breast cancer cells. In addition, CAV-1 significantly inhibited cell proliferation and modulated DTX-induced apoptosis through cell cycle arrest in the G2/M phase. The mechanisms underlying DTX-induced apoptosis differed in breast cancers according to the levels of CAV-1 expression. DTX robustly enhanced Bcl-2 inactivation by CAV-1 in MDA-MB-231 cells, while p53-mediated cell cycle arrest by DTX was more pronounced in CAV-1-low but p53-functional MCF-7 cells. In parallel with the data from breast cancer cell lines, CAV-1-transfected MCF-7 cells showed higher efficacy of DTX treatment in a xenograft model.
CONCLUSION
We clearly demonstrated cooperative effects between CAV-1 and DTX in mediating apoptosis, suggesting that the levels of CAV-1 expression might be an important indicator for DTX use in breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Koleske AJ, Baltimore D, Lisanti MP. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc Natl Acad Sci U S A. 1995; 92:1381–5.
Article2. Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and cancer metabolism in the tumor microenvironment: markers, models, and mechanisms. Annu Rev Pathol. 2012; 7:423–67.
Article3. Park SS, Kim JE, Kim YA, Kim YC, Kim SW. Caveolin-1 is down-regulated and inversely correlated with HER2 and EGFR expression status in invasive ductal carcinoma of the breast. Histopathology. 2005; 47:625–30.
Article4. Savage K, Lambros MB, Robertson D, Jones RL, Jones C, Mackay A, et al. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007; 13:90–101.5. Rao X, Evans J, Chae H, Pilrose J, Kim S, Yan P, et al. CpG island shore methylation regulates caveolin-1 expression in breast cancer. Oncogene. 2013; 32:4519–28.
Article6. Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and "fibroblast addiction" are new therapeutic targets for drug discovery. Cell Cycle. 2013; 12:2723–32.
Article7. Martins D, Beca FF, Sousa B, Baltazar F, Paredes J, Schmitt F. Loss of caveolin-1 and gain of MCT4 expression in the tumor stroma: key events in the progression from an in situ to an invasive breast carcinoma. Cell Cycle. 2013; 12:2684–90.
Article8. Simpkins SA, Hanby AM, Holliday DL, Speirs V. Clinical and functional significance of loss of caveolin-1 expression in breast cancer-associated fibroblasts. J Pathol. 2012; 227:490–8.
Article9. Lyseng-Williamson KA, Fenton C. Docetaxel: a review of its use in metastatic breast cancer. Drugs. 2005; 65:2513–31.10. Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, et al. Molecular predictors of efficacy of adjuvant weekly paclitaxel in early breast cancer. Breast Cancer Res Treat. 2010; 123:149–57.
Article11. Pusztai L, Jeong JH, Gong Y, Ross JS, Kim C, Paik S, et al. Evaluation of microtubule-associated protein-Tau expression as a prognostic and predictive marker in the NSABP-B 28 randomized clinical trial. J Clin Oncol. 2009; 27:4287–92.
Article12. Tabchy A, Valero V, Vidaurre T, Lluch A, Gomez H, Martin M, et al. Evaluation of a 30-gene paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide chemotherapy response predictor in a multicenter randomized trial in breast cancer. Clin Cancer Res. 2010; 16:5351–61.
Article13. Shajahan AN, Wang A, Decker M, Minshall RD, Liu MC, Clarke R. Caveolin-1 tyrosine phosphorylation enhances paclitaxel-mediated cytotoxicity. J Biol Chem. 2007; 282:5934–43.
Article14. Gligorov J, Lotz JP. Preclinical pharmacology of the taxanes: implications of the differences. Oncologist. 2004; 9 Suppl 2:3–8.
Article15. Shajahan AN, Dobbin ZC, Hickman FE, Dakshanamurthy S, Clarke R. Tyrosine-phosphorylated caveolin-1 (Tyr-14) increases sensitivity to paclitaxel by inhibiting BCL2 and BCLxL proteins via c-Jun N-terminal kinase (JNK). J Biol Chem. 2012; 287:17682–92.
Article16. Van den Eynden GG, Van Laere SJ, Van der Auwera I, Merajver SD, Van Marck EA, van Dam P, et al. Overexpression of caveolin-1 and -2 in cell lines and in human samples of inflammatory breast cancer. Breast Cancer Res Treat. 2006; 95:219–28.
Article17. Ma X, Liu L, Nie W, Li Y, Zhang B, Zhang J, et al. Prognostic role of caveolin in breast cancer: a meta-analysis. Breast. 2013; 22:462–9.
Article18. Thompson DE, Siwicky MD, Moorehead RA. Caveolin-1 expression is elevated in claudin-low mammary tumor cells. Cancer Cell Int. 2012; 12:6.
Article19. Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, et al. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004; 279:51630–46.20. Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res. 1996; 56:1253–5.21. Singel SM, Cornelius C, Batten K, Fasciani G, Wright WE, Lum L, et al. A targeted RNAi screen of the breast cancer genome identifies KIF14 and TLN1 as genes that modulate docetaxel chemosensitivity in triple-negative breast cancer. Clin Cancer Res. 2013; 19:2061–70.
Article22. Lavie Y, Fiucci G, Liscovitch M. Up-regulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem. 1998; 273:32380–3.
Article23. Ando T, Ishiguro H, Kimura M, Mitsui A, Mori Y, Sugito N, et al. The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol Rep. 2007; 18:601–9.
Article24. Ho CC, Kuo SH, Huang PH, Huang HY, Yang CH, Yang PC. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer. 2008; 59:105–10.
Article25. Yang CP, Galbiati F, Volonte D, Horwitz SB, Lisanti MP. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998; 439:368–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERRATUM: Caveolin-1 Modulates Docetaxel-Induced Cell Death in Breast Cancer Cell Subtypes through Different Mechanisms
- Cell Death Induction Mechanism of Non-small Cell Lung Cancer Cell Line, NCI-H1703 by Docetaxel
- Caveolin-1 is involved in high glucose accelerated human glomerular mesangial cell senescence
- Increased Expression of Caveolin-1 in Renal Cell Carcinoma
- Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells