Korean J Physiol Pharmacol.
2019 May;23(3):161-169. 10.4196/kjpp.2019.23.3.161.
Fumigaclavine C attenuates adipogenesis in 3T3-L1 adipocytes and ameliorates lipid accumulation in high-fat diet-induced obese mice
- Affiliations
-
- 1Key Laboratory for Processing of Sugar Resources of Guangxi Higher Education Institutes, Guangxi University of Science and Technology, Liuzhou 545006, Guangxi, China. yuwanguo@hotmail.com
- 2Guangxi Key Laboratory of Green Processing of Sugar Resources, Guangxi University of Science and Technology, Liuzhou 545006, Guangxi, China.
- 3Gastroenterology Department, Liuzhou General Hospital, Liuzhou 545006, Guangxi, China. heyun1984@hotmail.com
- KMID: 2443604
- DOI: http://doi.org/10.4196/kjpp.2019.23.3.161
Abstract
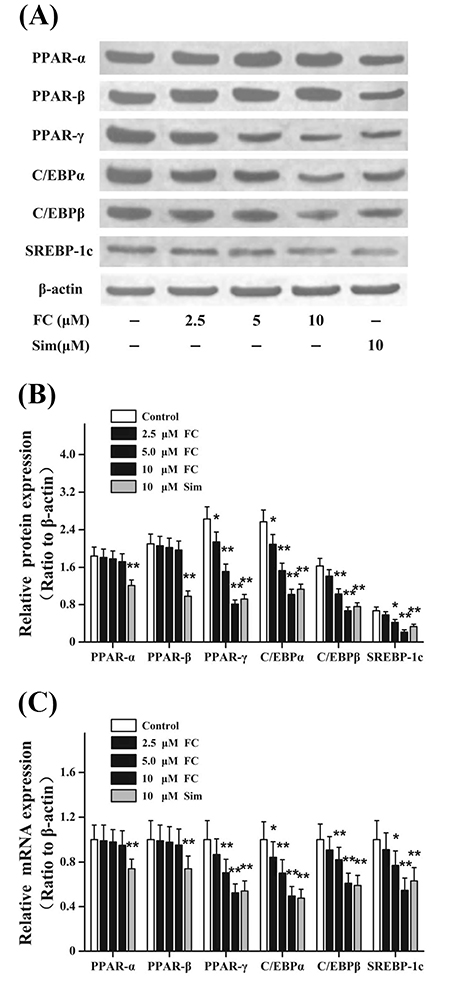
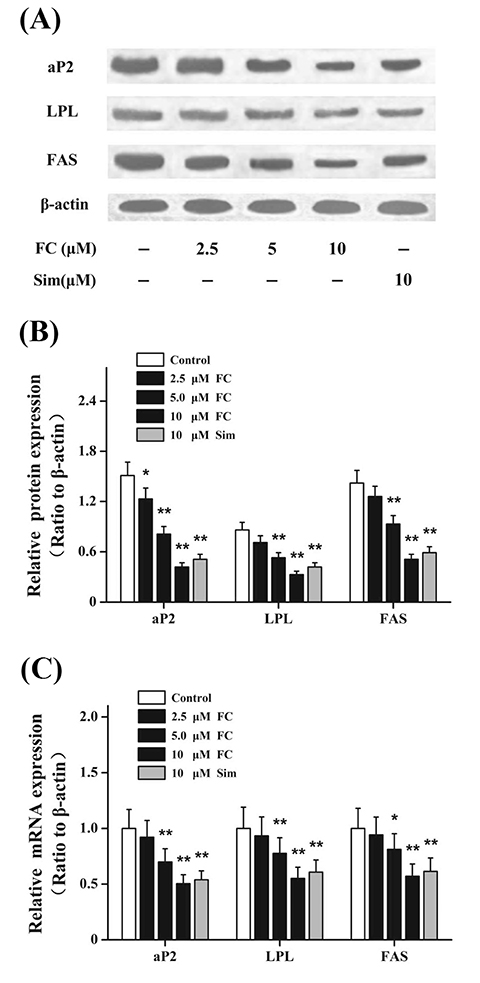
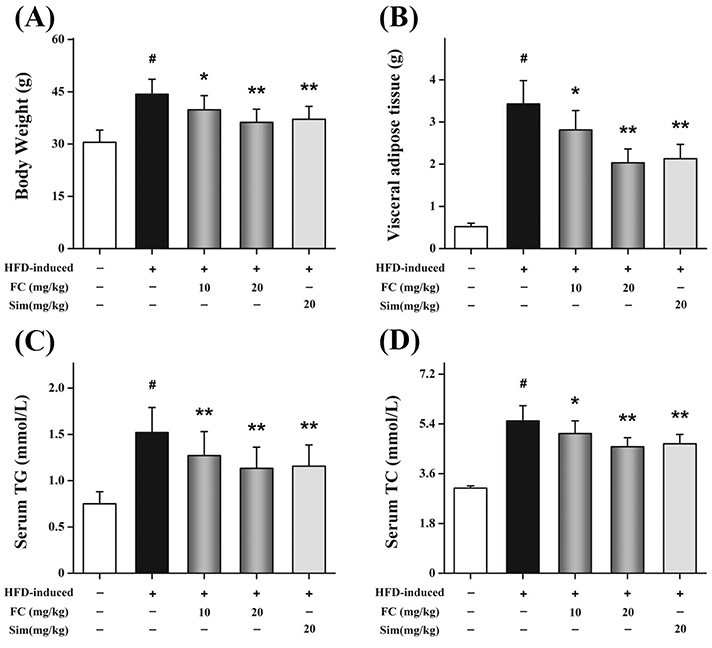
- Fumigaclavine C (FC), an active indole alkaloid, is obtained from endophytic Aspergillus terreus (strain No. FC118) by the root of Rhizophora stylosa (Rhizophoraceae). This study is designed to evaluate whether FC has anti-adipogenic effects in 3T3-L1 adipocytes and whether it ameliorates lipid accumulation in high-fat diet (HFD)-induced obese mice. FC notably increased the levels of glycerol in the culture supernatants and markedly reduced lipid accumulation in 3T3-L1 adipocytes. FC differentially inhibited the expressions of adipogenesis-related genes, including the peroxisome proliferator-activated receptor proteins, CCAAT/enhancer-binding proteins, and sterol regulatory element-binding proteins. FC markedly reduced the expressions of lipid synthesis-related genes, such as the fatty acid binding protein, lipoprotein lipase, and fatty acid synthase. Furthermore, FC significantly increased the expressions of lipolysis-related genes, such as the hormone-sensitive lipase, Aquaporin-7, and adipose triglyceride lipase. In HFD-induced obese mice, intraperitoneal injections of FC decreased both the body weight and visceral adipose tissue weight. FC administration significantly reduced lipid accumulation. Moreover, FC could dose-dependently and differentially regulate the expressions of lipid metabolism-related transcription factors. All these data indicated that FC exhibited anti-obesity effects through modulating adipogenesis and lipolysis.
Keyword
MeSH Terms
-
Adipocytes*
Adipogenesis*
Animals
Aspergillus
Body Weight
Carrier Proteins
Diet, High-Fat
Glycerol
Injections, Intraperitoneal
Intra-Abdominal Fat
Lipase
Lipolysis
Lipoprotein Lipase
Mice
Mice, Obese*
Peroxisomes
Rhizophoraceae
Sterol Esterase
Transcription Factors
Carrier Proteins
Glycerol
Lipase
Lipoprotein Lipase
Sterol Esterase
Transcription Factors
Figure
Reference
-
1. Gangadaran S, Cheema SK. A high fat diet enriched with sea cucumber gut powder provides cardio-protective and anti-obesity effects in C57BL/6 mice. Food Res Int. 2017; 99:799–806.
Article2. Imai N, Suzuki M, Ishizu Y, Kuzuya T, Honda T, Hayashi K, Ishigami M, Hirooka Y, Ishikawa T, Goto H, Fujimoto T. Hepatocytespecific depletion of ubiquitin regulatory X domain containing protein 8 accelerates fibrosis in a mouse non-alcoholic steatohepatitis model. Histochem Cell Biol. 2017; 148:219–227.
Article3. Yu WG, He H, Yao JY, Zhu YX, Lu YH. Dimethyl cardamonin exhibits anti-inflammatory Effects via interfering with the PI3K-PDK1-PKCα signaling pathway. Biomol Ther (Seoul). 2015; 23:549–556.
Article4. Chandrashekaran V, Seth RK, Dattaroy D, Alhasson F, Ziolenka J, Carson J, Berger FG, Kalyanaraman B, Diehl AM, Chatterjee S. HMGB1-RAGE pathway drives peroxynitrite signaling-induced IBD-like inflammation in murine nonalcoholic fatty liver disease. Redox Biol. 2017; 13:8–19.
Article5. Tian F, Wu CL, Yu BL, Liu L, Hu JR. Apolipoprotein O expression in mouse liver enhances hepatic lipid accumulation by impairing mitochondrial function. Biochem Biophys Res Commun. 2017; 491:8–14.
Article6. Hirotani Y, Fukamachi J, Ueyama R, Urashima Y, Ikeda K. Effects of capsaicin coadministered with eicosapentaenoic acid on obesity-related dysregulation in high-fat-fed mice. Biol Pharm Bull. 2017; 40:1581–1585.
Article7. Kang HH, Kim IK, Lee HI, Joo H, Lim JU, Lee J, Lee SH, Moon HS. Chronic intermittent hypoxia induces liver fibrosis in mice with diet-induced obesity via TLR4/MyD88/MAPK/NF-κB signaling pathways. Biochem Biophys Res Commun. 2017; 490:349–355.8. Pražienková V, Holubová M, Pelantová H, Bugáňová M, Pirník Z, Mikulášková B, Popelová A, Blechová M, Haluzík M, Železná B, Kuzma M, Kuneš J, Maletínská L. Impact of novel palmitoylated prolactin-releasing peptide analogs on metabolic changes in mice with diet-induced obesity. PLoS One. 2017; 12:e0183449.
Article9. Lee JH, Kang HS, Park HY, Moon YA, Kang YN, Oh BC, Song DK, Bae JH, Im SS. PPARα-dependent Insig2a overexpression inhibits SREBP-1c processing during fasting. Sci Rep. 2017; 7:9958.
Article10. Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015; 16:678–689.
Article11. Mota de Sá P, Richard AJ, Hang H, Stephens JM. Transcriptional regulation of adipogenesis. Compr Physiol. 2017; 7:635–674.12. Tang Q, Jiang S, Jia W, Shen D, Qiu Y, Zhao Y, Xue B, Li C. Zoledronic acid, an FPPS inhibitor, ameliorates liver steatosis through inhibiting hepatic de novo lipogenesis. Eur J Pharmacol. 2017; 814:169–177.
Article13. Hsiao PJ, Chiou HC, Jiang HJ, Lee MY, Hsieh TJ, Kuo KK. Pioglitazone enhances cytosolic lipolysis, β-oxidation and autophagy to ameliorate hepatic steatosis. Sci Rep. 2017; 7:9030.
Article14. Lass A, Zimmermann R, Oberer M, Zechner R. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog Lipid Res. 2011; 50:14–27.
Article15. Lebeck J. Metabolic impact of the glycerol channels AQP7 and AQP9 in adipose tissue and liver. J Mol Endocrinol. 2014; 52:R165–R178.
Article16. Yu WG, He H, Qian J, Lu YH. Dual role of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone in inhibiting high-mobility group box 1 secretion and blocking its pro-inflammatory activity in hepatic inflammation. J Agric Food Chem. 2014; 62:11949–11956.
Article17. Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS One. 2017; 12:e0183541.
Article18. Wang S, Huang Y, Xu H, Zhu Q, Lu H, Zhang M, Hao S, Fang C, Zhang D, Wu X, Wang X, Sheng J. Oxidized tea polyphenols prevent lipid accumulation in liver and visceral white adipose tissue in rats. Eur J Nutr. 2017; 56:2037–2048.
Article19. Wang Y, Zhu H, Tam NFY. Polyphenols, tannins and antioxidant activities of eight true mangrove plant species in South China. Plant Soil. 2014; 374:549–563.
Article20. Tan Y, Wu X, Sun J, Guo W, Gong F, Shao F, Tan T, Cao Y, Zheng B, Gu Y, Sun Y, Xu Q. A fumigaclavine C isostere alleviates Th1-mediated experimental colitis via competing with IFN-γ for binding to IFN-γ receptor 1. Biochem Pharmacol. 2017; 123:63–72.
Article21. Zhao Y, Liu J, Wang J, Wang L, Yin H, Tan R, Xu Q. Fumigaclavine C improves concanavalin A-induced liver injury in mice mainly via inhibiting TNF-α production and lymphocyte adhesion to extracellular matrices. J Pharm Pharmacol. 2004; 56:775–782.
Article22. Guo W, Hu S, Elgehama A, Shao F, Ren R, Liu W, Zhang W, Wang X, Tan R, Xu Q, Sun Y, Jiao R. Fumigaclavine C ameliorates dextran sulfate sodium-induced murine experimental colitis via NLRP3 inflammasome inhibition. J Pharmacol Sci. 2015; 129:101–106.
Article23. Yu W, Pan Z, Zhu Y, An F, Lu Y. Fumigaclavine C exhibits anti-inflammatory effects by suppressing high mobility group box protein 1 relocation and release. Eur J Pharmacol. 2017; 812:234–242.
Article24. Du RH, Li EG, Cao Y, Song YC, Tan RX. Fumigaclavine C inhibits tumor necrosis factor α production via suppression of toll-like receptor 4 and nuclear factor κB activation in macrophages. Life Sci. 2011; 89:235–240.
Article25. Du RH, Qin SY, Shi LS, Zhou ZQ, Zhu XY, Liu J, Tan RX, Cao W. Fumigaclavine C activates PPARγ pathway and attenuates atherogenesis in ApoE-deficient mice. Atherosclerosis. 2014; 234:120–128.
Article26. Fenni S, Hammou H, Astier J, Bonnet L, Karkeni E, Couturier C, Tourniaire F, Landrier JF. Lycopene and tomato powder supplementation similarly inhibit high-fat diet induced obesity, inflammatory response, and associated metabolic disorders. Mol Nutr Food Res. 2017; 61:1601083.
Article27. Yu WG, Qian J, Lu YH. Hepatoprotective effects of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone on CCl4-induced acute liver injury in mice. J Agric Food Chem. 2011; 59:12821–12829.
Article28. Veiga FMS, Graus-Nunes F, Rachid TL, Barreto AB, Mandarim-de-Lacerda CA, Souza-Mello V. Anti-obesogenic effects of WY14643 (PPAR-alpha agonist): hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice. Biochimie. 2017; 140:106–116.29. Liou CJ, Wu SJ, Chen LC, Yeh KW, Chen CY, Huang WC. Acacetin from traditionally used saussurea involucrata kar. et Kir. Suppressed adipogenesis in 3T3-L1 adipocytes and attenuated lipid accumulation in obese mice. Front Pharmacol. 2017; 8:589.
Article30. Hu YC, Zhang Z, Shi WG, Mi TY, Zhou LX, Huang N, Hoptroff M, Lu YH. 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone promoted glucose uptake and imposed a paradoxical effect on adipocyte differentiation in 3T3-L1 cells. J Agric Food Chem. 2014; 62:1898–1904.
Article31. Singh SP, Sashidhara KV. Lipid lowering agents of natural origin: an account of some promising chemotypes. Eur J Med Chem. 2017; 140:331–348.
Article32. Panahi Y, Ahmadi Y, Teymouri M, Johnston TP, Sahebkar A. Curcumin as a potential candidate for treating hyperlipidemia: a review of cellular and metabolic mechanisms. J Cell Physiol. 2018; 233:141–152.
Article33. Xiao S, Yu R, Ai N, Fan X. Rapid screening natural-origin lipase inhibitors from hypolipidemic decoctions by ultrafiltration combined with liquid chromatography-mass spectrometry. J Pharm Biomed Anal. 2015; 104:67–74.
Article34. Zhao SP, Li R, Dai W, Yu BL, Chen LZ, Huang XS. Xuezhikang contributes to greater triglyceride reduction than simvastatin in hypertriglyceridemia rats by up-regulating apolipoprotein A5 via the PPARα signaling pathway. PLoS One. 2017; 12:e0184949.
Article35. Tarantino N1, De Gennaro L, Correale M, Guastafierro F, Gaglione A, Di Biase M, Brunetti ND. Fenofibrate/simvastatin fixed-dose combination in the treatment of mixed dyslipidemia: safety, efficacy, and place in therapy. Vasc Health Risk Manag. 2017; 13:29–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
- Capsanthin Inhibits both Adipogenesis in 3T3-L1 Preadipocytes and Weight Gain in High-Fat Diet-Induced Obese Mice
- Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet
- Expression of eotaxin in 3T3-L1 adipocytes and the effects of weight loss in high-fat diet induced obese mice
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3