Nutr Res Pract.
2011 Feb;5(1):11-19.
Expression of eotaxin in 3T3-L1 adipocytes and the effects of weight loss in high-fat diet induced obese mice
- Affiliations
-
- 1School of Life Science, Handong Global University, Heunghae-eup, Buk-gu, Pohang-si, Gyeongbuk 791-708, Korea. msdo@handong.edu
Abstract
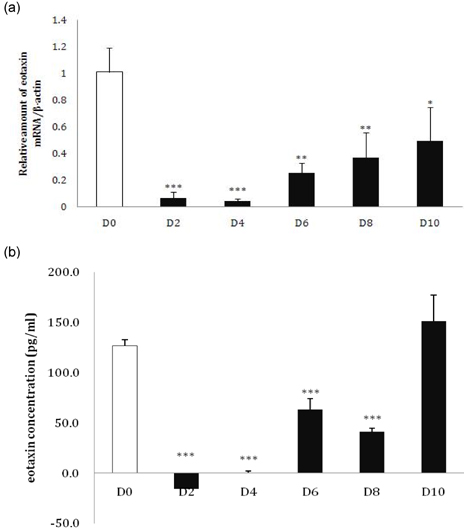
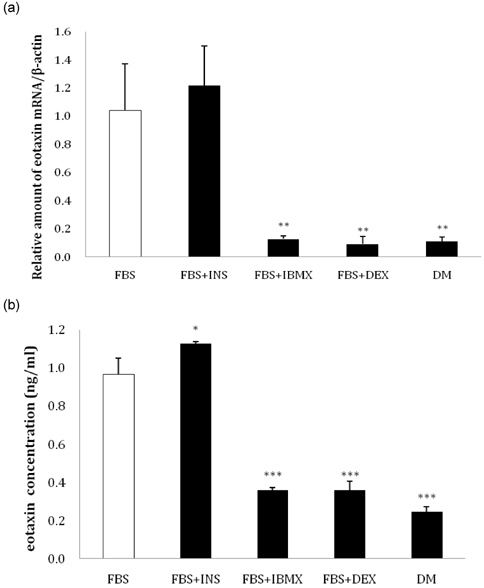
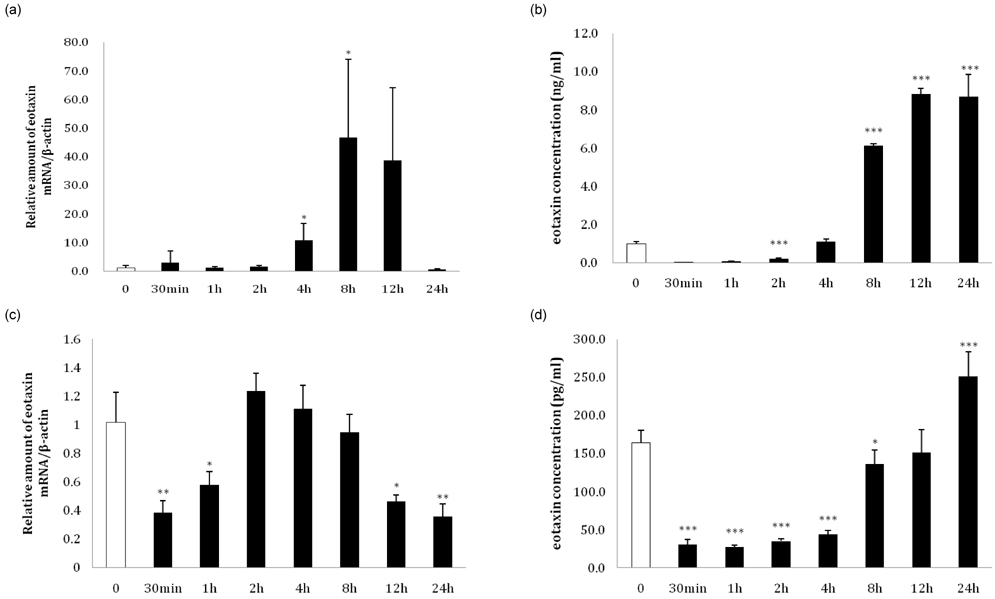
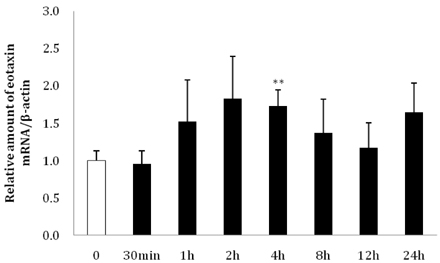
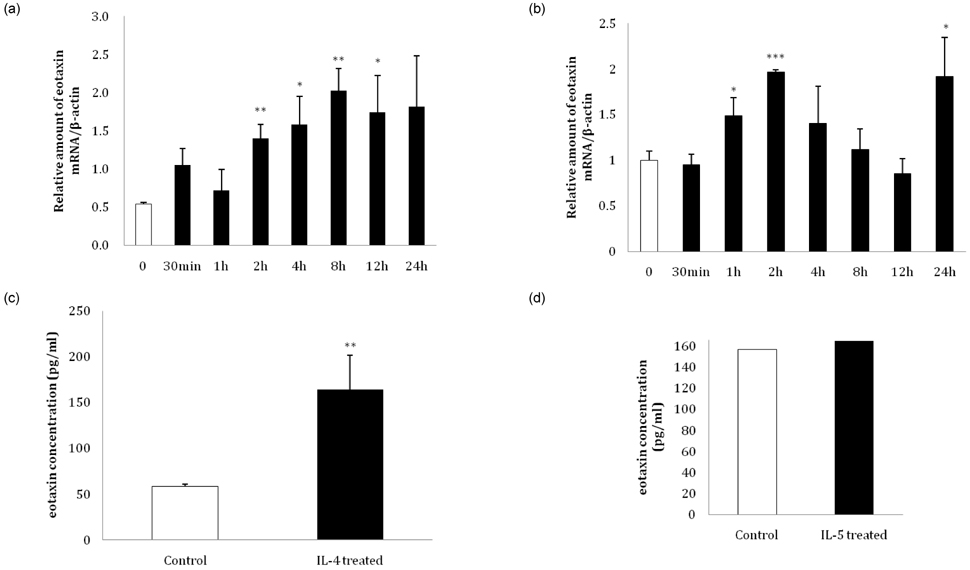
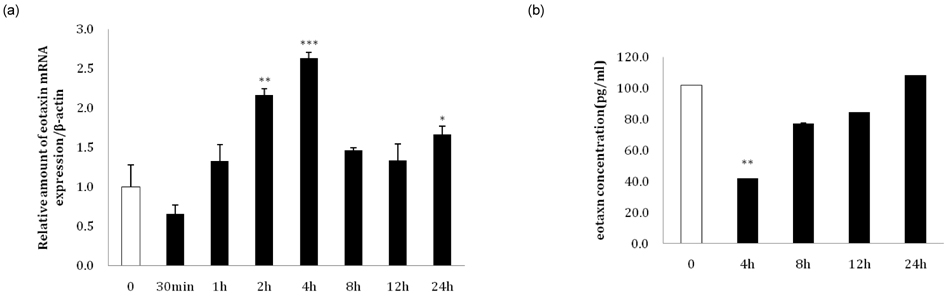
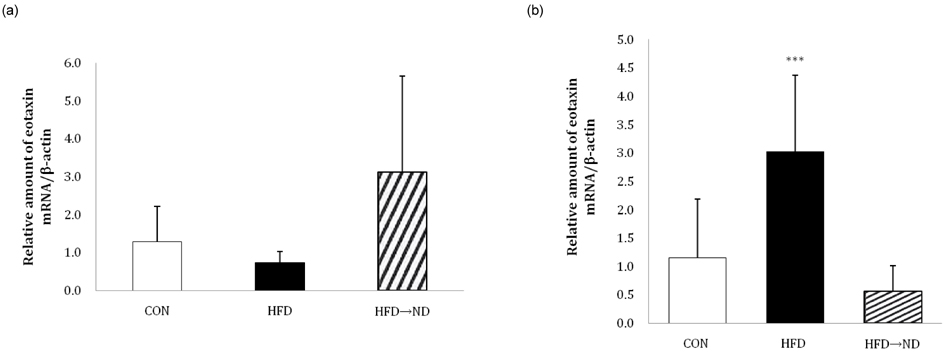
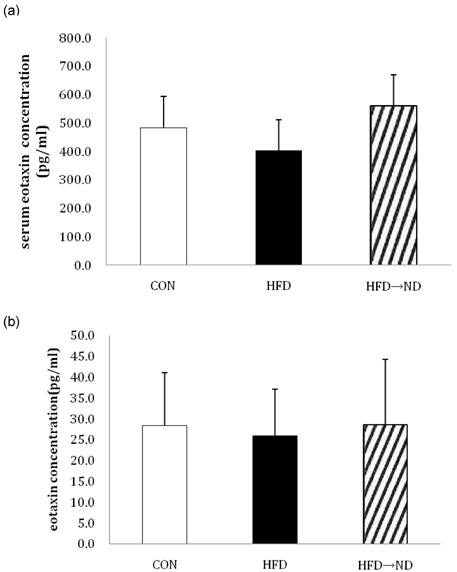
- Eotaxin is an important inflammatory chemokine in eosinophil chemotaxis and activation and, thus, is implicated in asthma. Recently, obesity was associated with an increased prevalence of asthma, but the relationship between obesity and eotaxin expression has only been partially understood in obese mice and human studies. Therefore, we studied the expression patterns of eotaxin in 3T3-L1 preadipocytes/adipocytes to determine whether eotaxin levels are influenced by body weight gain and/or reduction in diet-induced obese mice. First, we investigated eotaxin expression during differentiation in 3T3-L1 adipocytes. Then, we treated 3T3-L1 preadipocytes/adipocytes with tumor necrosis factor-alpha (TNF-alpha), eotaxin, interleukin (IL)-4, IL-5, or leptin. To examine the effects of weight loss in high-fat diet induced obese mice, we fed C57BL/6 mice a high-fat diet or a normal diet for 26 weeks. Then, half of the high-fat diet group were fed a normal diet until 30 weeks to reduce weight. Epididymal adipose tissue, visceral adipose tissue, serum, and bronchoalveolar fluid of mice were examined for eotaxin expression. The results showed that eotaxin expression levels increased with adipocyte differentiation and that more eotaxin was expressed when the cells were stimulated with TNF-alpha, eotaxin, IL-4, IL-5, or leptin. An in vivo study showed that eotaxin levels were reduced in visceral adipose tissues when high-fat diet fed mice underwent weight loss. Taken together, these results indicate a close relationship between eotaxin expression and obesity as well as weight loss, thus, they indirectly show a relation to asthma.
Keyword
MeSH Terms
Figure
Reference
-
1. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005. 115:911–919.
Article2. Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro, and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993. 197:1167–1172.3. Kitaura M, Nakajima T, Imai T, Harada S, Combadiere C, Tiffany HL, Murphy PM, Yoshie O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. 1996. 271:7725–7730.
Article4. Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol. 2008. 104:1727–1735.
Article5. Li D, Wang D, Griffiths-Johnson DA, Wells TN, Williams TJ, Jose PJ, Jeffery PK. Eotaxin protein and gene expression in guinea-pig lungs: constitutive expression and upregulation after allergen challenge. Eur Respir J. 1997. 10:1946–1954.
Article6. Bartels J, Schlüter C, Richter E, Noso N, Kulke R, Christophers E, Schröder JM. Human dermal fibroblasts express eotaxin: molecular cloning, mRNA epression, andidentification of eotaxin sequence variants. Biochem Biophys Res Commun. 1996. 225:1045–1051.
Article7. Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and obesity. J Clin Endocrinol Metab. 2006. 91:256–261.
Article8. Hashimoto I, Wada J, Hida A, Baba M, Miyatake N, Eguchi J, Shikata K, Makino H. Elevated serum monocyte chemoattractant protein-4 and chronic inflammation in overweight subjects. Obesity (Silver Spring). 2006. 14:799–811.
Article9. Beckett WS, Jacobs DR Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001. 164:2045–2050.
Article10. Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002. 122:1256–1263.
Article11. Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999. 54:396–402.
Article12. Huang SL, Shiao G, Chou P. Association between body mass index and allergy in teenage girls in Taiwan. Clin Exp Allergy. 1999. 29:323–329.
Article13. Xu B, Jarvelin MR, Pekkanen J. Body build and atopy. J Allergy Clin Immunol. 2000. 105:393–394.
Article14. Stenius-Aarniala B, Poussa T, Kvarnström J, Grönlund EL, Ylikahri M, Mustajoki P. Immediate and long term effects of weight reduction in obese people with asthma: randomized controlled study. BMJ. 2000. 320:827–832.
Article15. Choi KM, Kim JH, Cho GJ, Baik SH, Park HS, Kim SM. Effect of exercise training on plasma visfatin and eotaxin levels. Eur J Endocrinol. 2007. 157:437–442.
Article16. Do MS, Jeong HS, Choi BH, Hunter L, Langley S, Pazmany L, Trayhurn P. Inflammatory gene expression patterns revealed by DNA microarray analysis in TNF-alpha-treated SGBS human adipocytes. Yonsei Med J. 2006. 47:729–736.
Article17. Poulain-Godefroy O, Froguel P. Preadipocyte response and impairment of differentiation in an inflammatory environment. Biochem Biophys Res Commun. 2007. 356:662–667.
Article18. Johnston RA, Zhu M, Rivera-Sanchez YM, Lu FL, Theman TA, Flynt L, Shore SA. Allergic airway responses in obese mice. Am J Respir Crit Care Med. 2007. 176:650–658.
Article19. Calixto MC, Lintomen L, Schenka A, Saad MJ, Zanesco A, Antunes E. Obesity enhances eosinophilic inflammation in a murine model of allergic asthma. Br J Pharmacol. 2010. 159:617–625.
Article20. Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005. 115:103–109.
Article21. Ntambi JM, Kim YC. Adipocyte differentiation and gene expression. J Nutr. 2000. 130:3122S–3126S.
Article22. Suzuki T, Arakawa H, Mizuno T, Muramatsu K, Tadaki H, Takizawa T, Mochizuki H, Tokuyama K, Matsukura S, Morikawa A. Differential regulation of eotaxin expression by dexamethasone in normal human Lung fibroblasts. Am J Respir Cell Mol Biol. 2008. 38:707–714.
Article23. Bjerke T, Gaustadnes M, Nielsen S, Schiøtz PO, Rudiger N, Reimert CM, Dahl R, Christensen I, Poulsen LK. Human blood eosinophils produce and secrete interleukin 4. Respir Med. 1996. 90:271–277.
Article24. Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994. 179:703–708.
Article25. Shore SA, Rivera-Sanchez YM, Schwartzman IN, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003. 95:938–945.
Article26. Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008. 93:3215–3221.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Capsanthin Inhibits both Adipogenesis in 3T3-L1 Preadipocytes and Weight Gain in High-Fat Diet-Induced Obese Mice
- The protective effects of steamed ginger on adipogenesis in 3T3-L1 cells and adiposity in diet-induced obese mice
- The anti-obesity effect of Lethariella cladonioides in 3T3-L1 cells and obese mice
- B-cell-activating factor is a regulator of adipokines and a possible mediator between adipocytes and macrophages
- Arctiin inhibits adipogenesis in 3T3-L1 cells and decreases adiposity and body weight in mice fed a high-fat diet