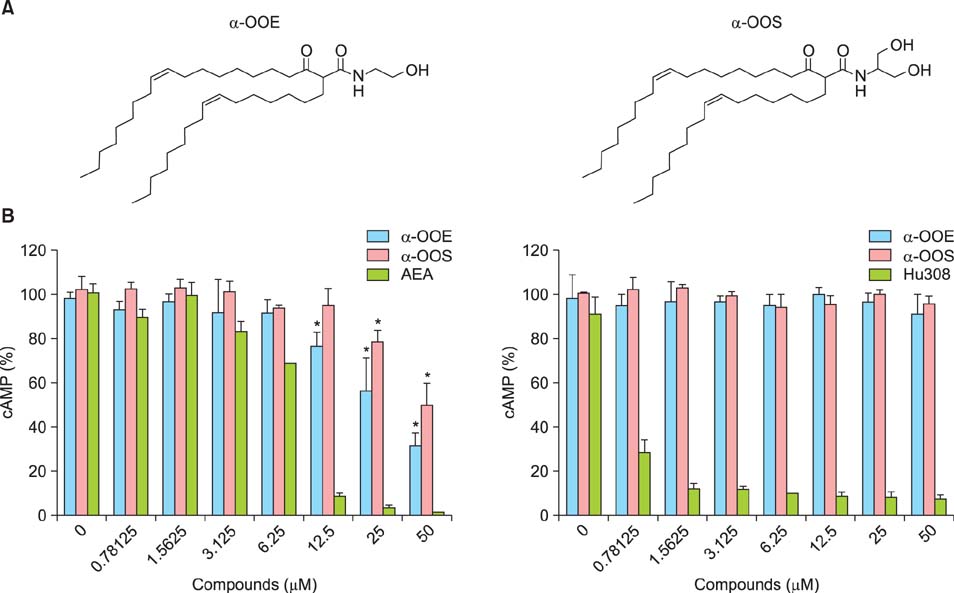
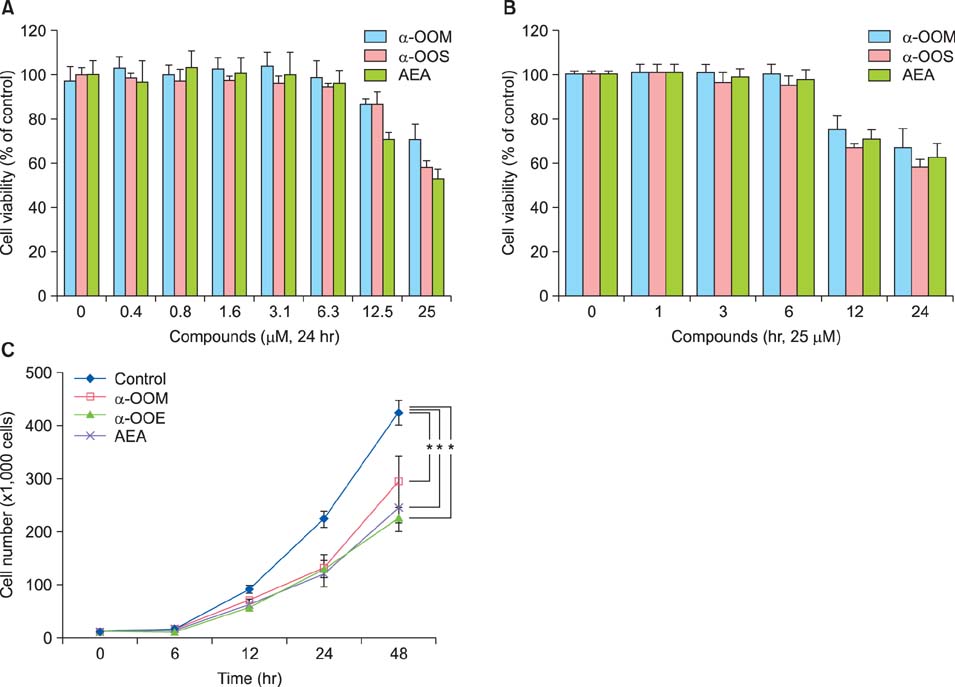
Ann Dermatol.
2016 Feb;28(1):22-29. 10.5021/ad.2016.28.1.22.
Selective Cannabinoid Receptor-1 Agonists Regulate Mast Cell Activation in an Oxazolone-Induced Atopic Dermatitis Model
- Affiliations
-
- 1Department of Cosmetic Science and Technology, Seowon University, Cheongju, Korea. sinope530@daum.net
- 2CRID Center, NeoPharm Co., Ltd., Daejeon, Korea.
- 3Department of Dermatology, Atopy and Asthma Center and Seoul Medical Research Institute, Seoul Medical Center, Seoul, Korea.
- 4Department of Dermatology, Dankook University Medical College, Cheonan, Korea.
- 5Department of Dermatology, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2429478
- DOI: http://doi.org/10.5021/ad.2016.28.1.22
Abstract
- BACKGROUND
Many inflammatory mediators, including various cytokines (e.g. interleukins and tumor necrosis factor [TNF]), inflammatory proteases, and histamine are released following mast cell activation. However, the endogenous modulators for mast cell activation and the underlying mechanism have yet to be elucidated. Endogenous cannabinoids such as palmitoylethanolamide (PEA) and N-arachidonoylethanolamine (anandamide or AEA), were found in peripheral tissues and have been proposed to possess autacoid activity, implying that cannabinoids may downregulate mast cell activation and local inflammation.
OBJECTIVE
In order to investigate the effect of cannabinoid receptor-1 (CB1R) agonists on mast cell activation, AEA-derived compounds were newly synthesized and evaluated for their effect on mast cell activation.
METHODS
The effects of selected compounds on FcepsilonRI-induced histamine and beta-hexosaminidase release were evaluated in a rat basophilic leukemia cell line (RBL-2H3). To further investigate the inhibitory effects of CB1R agonist in vivo, an oxazolone-induced atopic dermatitis mouse model was exploited.
RESULTS
We found that CB1R inhibited the release of inflammatory mediators without causing cytotoxicity in RBL-2H3 cells and that CB1R agonists markedly and dose-dependently suppressed mast cell proliferation indicating that CB1R plays an important role in modulating antigen-dependent immunoglobulin E (IgE)-mediated mast cell activation. We also found that topical application of CB1R agonists suppressed the recruitment of mast cells into the skin and reduced the level of blood histamine.
CONCLUSION
Our results indicate that CB1R agonists down-regulate mast cell activation and may be used for relieving inflammatory symptoms mediated by mast cell activation, such as atopic dermatitis, psoriasis, and contact dermatitis.
MeSH Terms
-
Animals
Basophils
beta-N-Acetylhexosaminidases
Cannabinoid Receptor Agonists
Cannabinoids
Cell Line
Cytokines
Dermatitis, Atopic*
Dermatitis, Contact
Histamine
Immunoglobulin E
Immunoglobulins
Inflammation
Interleukins
Leukemia
Mast Cells*
Mice
Peptide Hydrolases
Psoriasis
Rats
Skin
Tumor Necrosis Factor-alpha
Cannabinoid Receptor Agonists
Cannabinoids
Cytokines
Histamine
Immunoglobulin E
Immunoglobulins
Interleukins
Peptide Hydrolases
Tumor Necrosis Factor-alpha
beta-N-Acetylhexosaminidases
Figure
Reference
-
1. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964; 86:1646–1647.
Article2. Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997; 74:129–180.
Article3. Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002; 54:161–202.
Article4. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990; 346:561–564.
Article5. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993; 365:61–65.
Article6. Childers SR, Deadwyler SA. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem Pharmacol. 1996; 52:819–827.
Article7. Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005; 38:177–188.
Article8. Buckley NE. The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol. 2008; 153:309–318.
Article9. Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, et al. The cannabinoid system and immune modulation. J Leukoc Biol. 2003; 74:486–496.
Article10. Derocq JM, Ségui M, Marchand J, Le Fur G, Casellas P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 1995; 369:177–182.
Article11. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010; 125:2 Suppl 2. S73–S80.
Article12. Aloe L, Leon A, Levi-Montalcini R. A proposed autacoid mechanism controlling mastocyte behaviour. Agents Actions. 1993; 39(Spec No):C145–C147.
Article13. Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995; 92:3376–3380.
Article14. Sun N, Zhou C, Zhou X, Sun L, Che H. Use of a rat basophil leukemia (RBL) cell-based immunological assay for allergen identification, clinical diagnosis of allergy, and identification of anti-allergy agents for use in immunotherapy. J Immunotoxicol. 2015; 12:199–205.
Article15. Maeyama K, Hohman RJ, Metzger H, Beaven MA. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J Biol Chem. 1986; 261:2583–2592.
Article16. Samson MT, Small-Howard A, Shimoda LM, Koblan-Huberson M, Stokes AJ, Turner H. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. J Immunol. 2003; 170:4953–4962.
Article17. Sugawara K, Bíró T, Tsuruta D, Tóth BI, Kromminga A, Zákány N, et al. Endocannabinoids limit excessive mast cell maturation and activation in human skin. J Allergy Clin Immunol. 2012; 129:726–738.e8.
Article18. Lee HJ, Jung M, Kim JH, Yoon NY, Choi EH. The effect of adipose-derived stem cell-cultured media on oxazolone treated atopic dermatitis-like murine model. Ann Dermatol. 2012; 24:181–188.
Article19. Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009; 39:1786–1800.
Article20. Lambert DM, DiPaolo FG, Sonveaux P, Kanyonyo M, Govaerts SJ, Hermans E, et al. Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB(2) cannabinoid, as potential ligands for the cannabinoid receptors. Biochim Biophys Acta. 1999; 1440:266–274.
Article21. Bueb JL, Lambert DM, Tschirhart EJ. Receptor-independent effects of natural cannabinoids in rat peritoneal mast cells in vitro. Biochim Biophys Acta. 2001; 1538:252–259.
Article22. Oka S, Wakui J, Ikeda S, Yanagimoto S, Kishimoto S, Gokoh M, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J Immunol. 2006; 177:8796–8805.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- FFA2 Activation Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis in Mice
- The Effect of Adipose-Derived Stem Cell-Cultured Media on Oxazolone Treated Atopic Dermatitis-Like Murine Model
- The Cannabinoid Agonist WIN55,212-2 Suppresses Opioid-induced Pruritus in Mice
- In Vitro and In Vivo Validation of EP2-Receptor Agonism to Selectively Achieve Inhibition of Mast Cell Activity
- Selective Inhibition of β-Catenin/Co-Activator Cyclic AMP Response Element-Binding Protein-Dependent Signaling Prevents the Emergence of Hapten-Induced Atopic Dermatitis-Like Dermatitis