Lab Anim Res.
2018 Sep;34(3):118-125. 10.5625/lar.2018.34.3.118.
Successful development of squamous cell carcinoma and hyperplasia in RGEN-mediated p27 KO mice after the treatment of DMBA and TPA
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Korea. dyhwang@pusan.ac.kr
- 2Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital, Korea.
- 3Department of Biochemistry, College of Life Science and Biotechnology, Yonsei University, Korea.
- KMID: 2420824
- DOI: http://doi.org/10.5625/lar.2018.34.3.118
Abstract
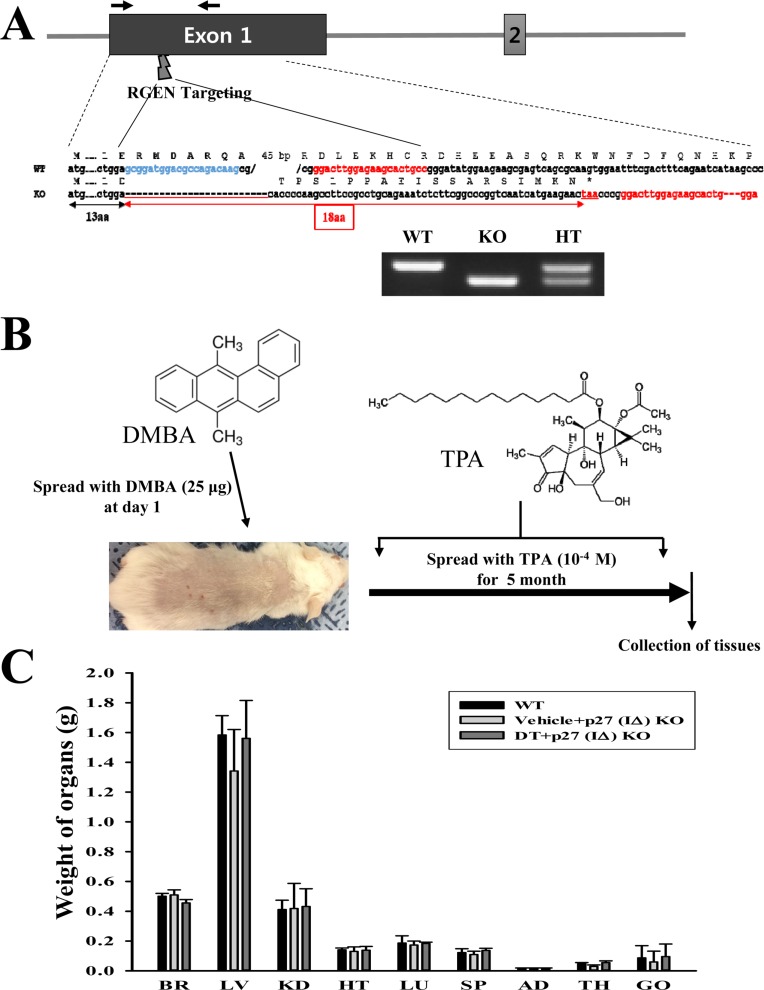
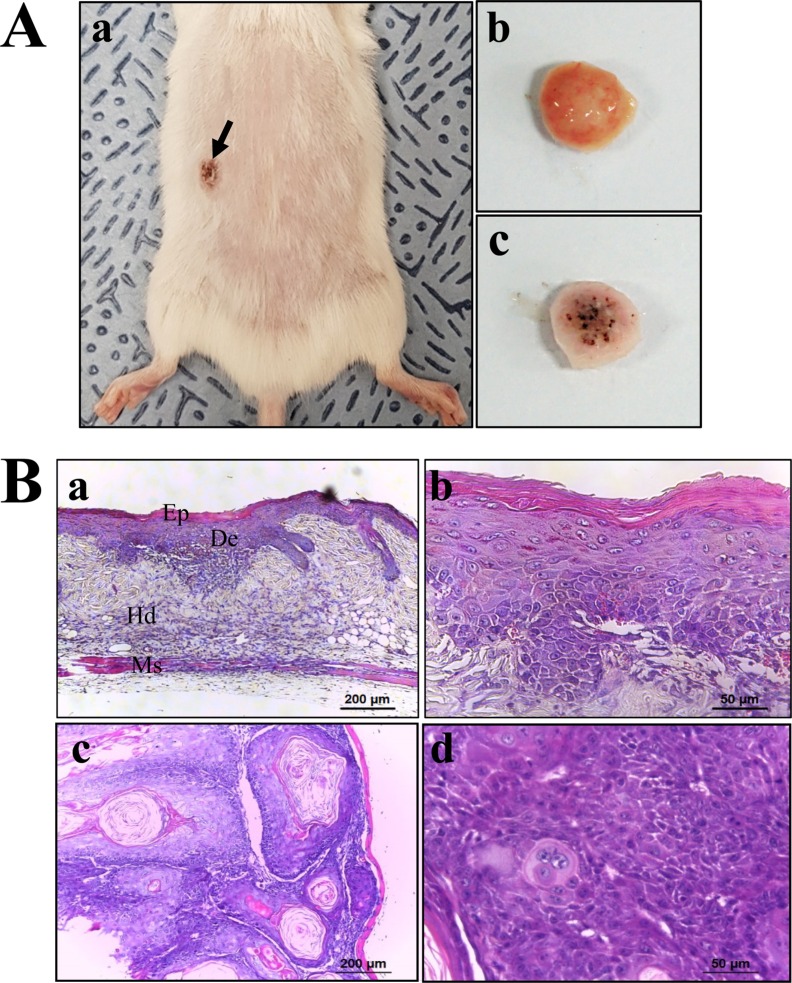
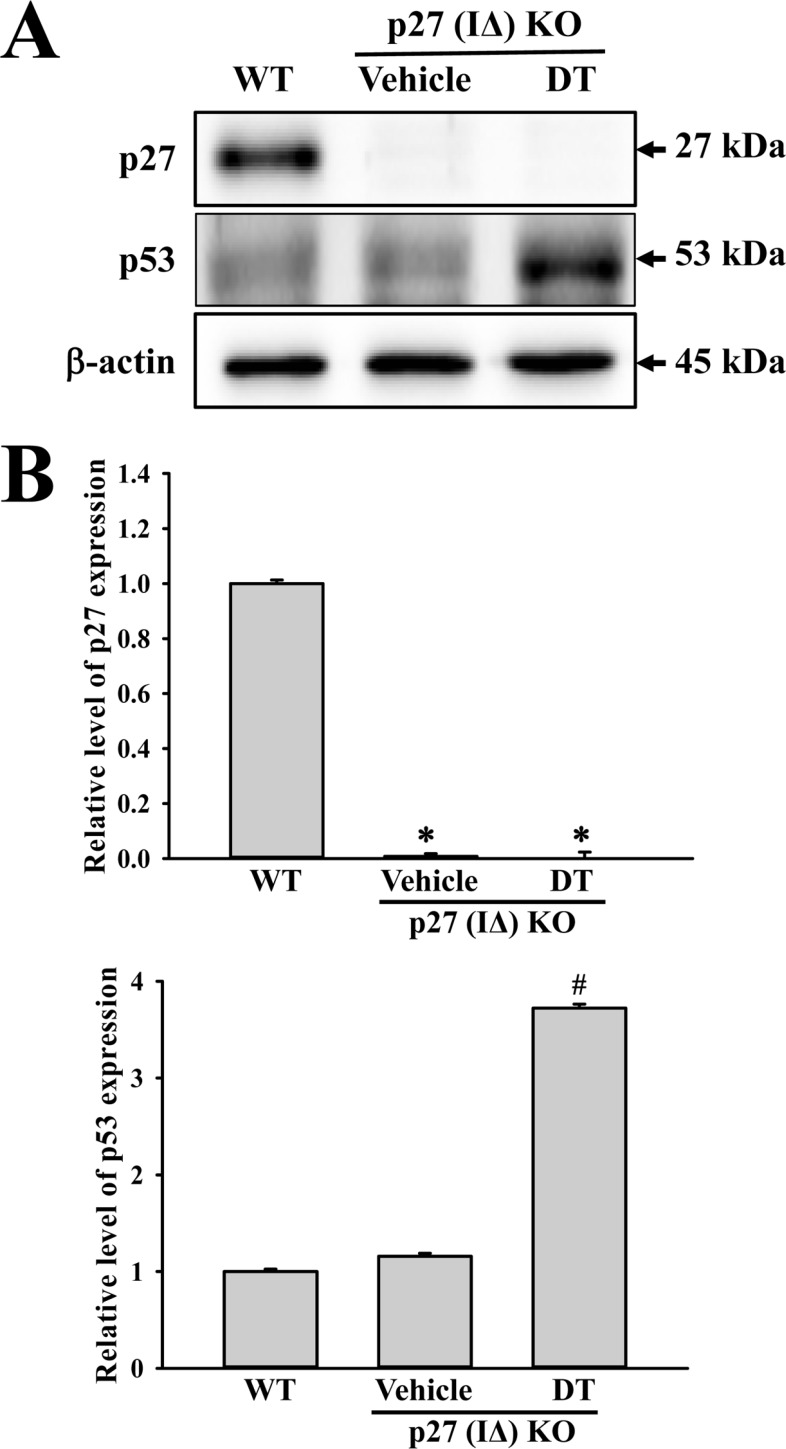
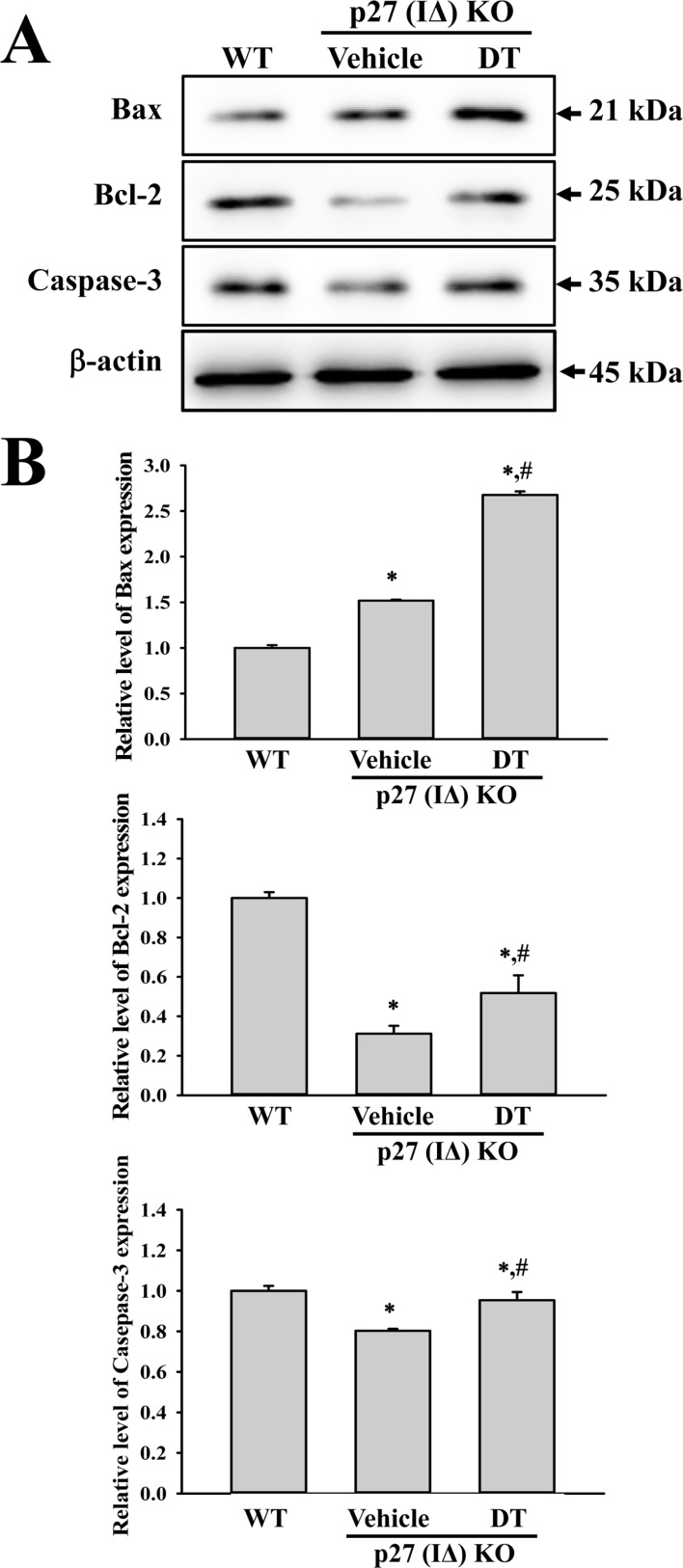
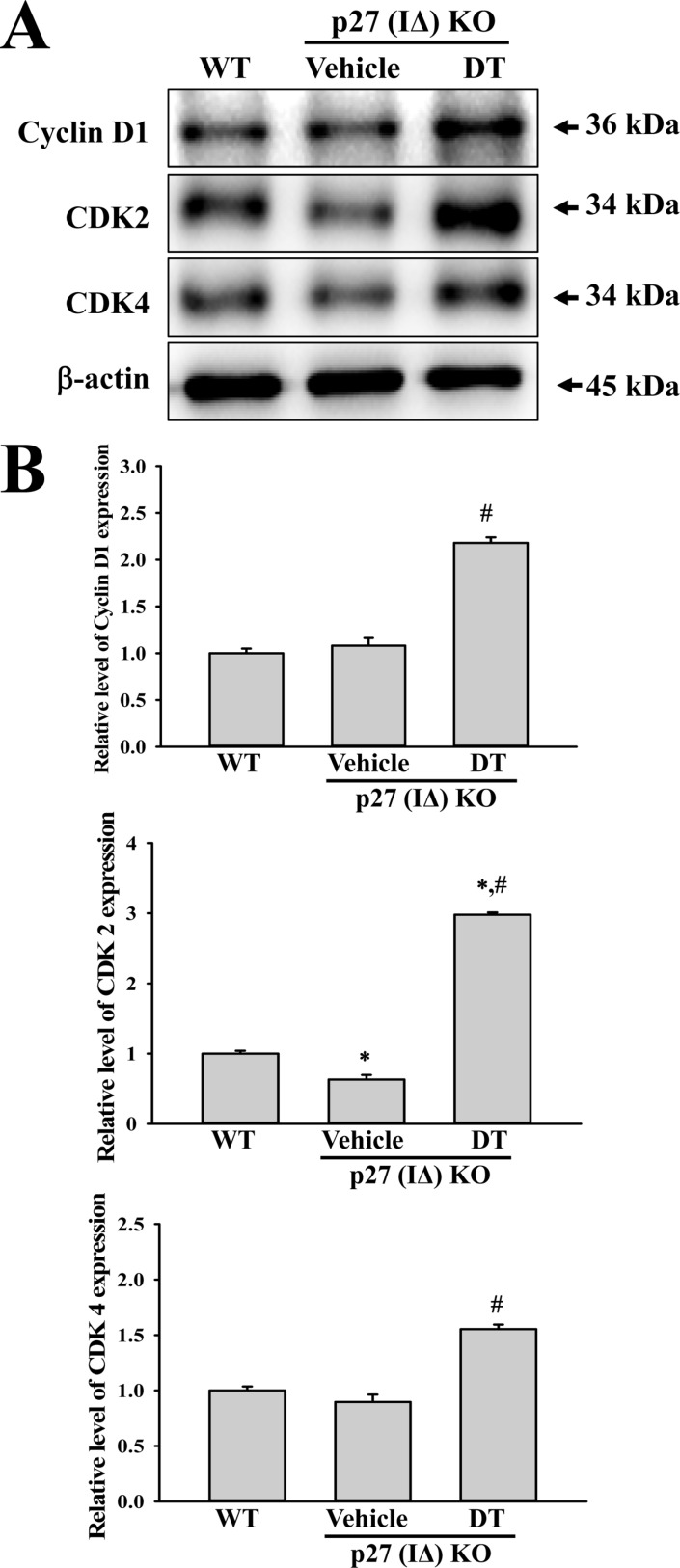
- To evaluate the carcinogenicity of p27 knockout (KO) mice with RNA-guided endonuclease (RGENs)-mediated p27 mutant exon I gene (IΔ), alterations in the carcinogenic phenotypes including tumor spectrum, tumor suppressor proteins, apoptotic proteins and cell cycle regulators were observed in p27 (IΔ) KO mice after treatment with 7,12-Dimethylbenz[a]anthracene (DMBA) and 12-O-tetradecanoylphorbol-13-acetate (TPA)(DT) for 5 months. The target region (544~571 nt) in exon I of the p27 gene was successfully disrupted in p27 (IΔ) KO mice using the RGEN-induced non-homologous end joining (NHEJ) technique. After DT exposure for 5 months, a few solid tumors (identified as squamous cell carcinoma) developed on the surface of back skin of DT-treated p27 (IΔ) KO mice. Also, squamous cell hyperplasia with chronic inflammation was detected in the skin dermis of DT-treated p27 (IΔ) KO mice, while the Vehicle+p27 (IΔ) KO mice and WT mice maintained their normal histological skin structure. A significant increase was observed in the expression levels of tumor suppressor protein (p53), apoptotic proteins (Bax, Bcl-2 and Caspase-3) and cell-cycle regulator proteins (Cyclin D1, CDK2 and CDK4) in the skin of DT-treated p27 (IΔ) KO mice, although their enhancement ratio was varied. Taken together, the results of the present study suggest that squamous cell carcinoma and hyperplasia of skin tissue can be successfully developed in new p27 (IΔ) KO mice produced by RGEN-induced NHEJ technique following DT exposure for 5 months.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Generation of knockout mouse models of cyclin-dependent kinase inhibitors by engineered nuclease-mediated genome editing
Bo Min Park, Jae-il Roh, Jaehoon Lee, Han-Woong Lee
Lab Anim Res. 2018;34(4):264-269. doi: 10.5625/lar.2018.34.4.264.CRISPR/Cas9-mediated generation of a
Plac8 knockout mouse model
HyunJeong Lee, Joo-Il Kim, Jin-Sung Park, Jae-il Roh, Jaehoon Lee, Byeong-Cheol Kang, Han-Woong Lee
Lab Anim Res. 2018;34(4):279-287. doi: 10.5625/lar.2018.34.4.279.
Reference
-
1. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999; 13(12):1501–1512. PMID: 10385618.
Article2. Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massagué J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994; 78(1):59–66. PMID: 8033212.3. Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994; 78(1):67–74. PMID: 8033213.
Article4. Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T. p27 expression and gastric carcinoma. Nat Med. 1997; 3(6):593. PMID: 9176477.
Article5. Kim DH, Lee HI, Nam ES, Shin HS, Sohn JH, Park CH, Yoon DS, Song SY, Park YE. Reduced expression of the cell-cycle inhibitor p27Kip1 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2000; 36(3):245–251. PMID: 10692028.6. Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998; 396(6707):177–180. PMID: 9823898.7. Philipp J, Vo K, Gurley KE, Seidel K, Kemp CJ. Tumor suppression by p27Kip1 and p21Cip1 during chemically induced skin carcinogenesis. Oncogene. 1999; 18(33):4689–4698. PMID: 10467416.8. Philipp-Staheli J, Kim KH, Payne SR, Gurley KE, Liggitt D, Longton G, Kemp CJ. Pathway-specific tumor suppression: Reduction of p27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer cell. 2002; 1(4):355–368. PMID: 12086850.9. Ogawa K, Murasaki T, Sugiura S, Nakanishi M, Shirai T. Organ differences in the impact of p27Kip1 deficiency on carcinogenesis induced by N-methyl-N-nitrosourea. J Appl Toxicol. 2013; 33(6):471–479. PMID: 22183835.10. Hikosaka A, Ogawa K, Sugiura S, Asamoto M, Takeshita F, Sato SY, Nakanishi M, Kohri K, Shirai T. Susceptibility of p27Kip1 knockout mice to urinary bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine may not simply be due to enhanced proliferation. Int J Cancer. 2008; 122(6):1222–1228. PMID: 18027869.11. Ellis FH Jr, Xu X, Kulke MH, LoCicero J 3rd, Loda M. Malignant transformation of the esophageal mucosa is enhanced in p27 knockout mice. J Thorac Cardiovasc Surg. 2001; 122(4):809–814. PMID: 11581618.
Article12. Guo J, Ma Q, Zhou X, Fan P, Shan T, Miao D. Inactivation of p27Kip1 promotes chemical hepatocarcinogenesis through enhancing inflammatory cytokine secretion and STAT3 signaling activation. J Cell Physiol. 2013; 228(10):1967–1976. PMID: 23460367.13. Fero ML, Rivkin M, Tasch M, Porter P, Carow CE, Firpo E, Polyak K, Tsai LH, Broudy V, Perlmutter RM, Kaushansky K, Roberts JM. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell. 1996; 85(5):733–744. PMID: 8646781.14. Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama K. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996; 85(5):707–720. PMID: 8646779.15. Kemp CJ. Animal models of chemical carcinogenesis: driving breakthroughs in cancer research for 100 years. Cold Spring Harb Protoc. 2015; 2015(10):865–874. PMID: 26430259.
Article16. Harvey M, McArthur MJ, Montgomery CA Jr, Butel JS, Bradley A, Donehower LA. Spontaneous and carcinogen-induced tumorigenesis in p53-deficient mice. Nat Genet. 1993; 5(3):225–229. PMID: 8275085.
Article17. Kemp CJ, Wheldon T, Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet. 1994; 8(1):66–69. PMID: 7987394.
Article18. Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994; 372(6508):773–776. PMID: 7997263.
Article19. Kiyokawa H, Kineman RD, Manova-Todorova KO, Soares VC, Hoffman ES, Ono M, Khanam D, Hayday AC, Frohman LA, Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell. 1996; 85(5):721–732. PMID: 8646780.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor associated proteins of rat skin tumor induced by 7,12-dimethylbenz[a]anthracene
- Effects of p27 Overexpression on Head and Neck Squamous Cell Carcinoma Cell Lines
- Pseudoepitheliomatous Hyperplasia Mimicking Esophageal Squamous Cell Carcinoma in a Patient with Lye-induced Esophageal Stricture
- Squamous Cell Carcinoma Arising from Chronic Osteomyelitis: A report of four Cases
- Correlation of Expression of galectin-3, skp2, p27 and cyclin D1 in Benign and Malignant Thyroid Lesions