J Korean Neurosurg Soc.
2016 Nov;59(6):544-550. 10.3340/jkns.2016.59.6.544.
Proteomic Analysis of a Rat Cerebral Ischemic Injury Model after Human Cerebral Endothelial Cell Transplantation
- Affiliations
-
- 1Department of Neurosurgery, Gwangju Christian Hospital, Gwangju, Korea.
- 2Department of Forensic Medicine, Chonnam National University Medical School, Gwangju, Korea. veritas@jnu.ac.kr
- 3Department of Nuclear Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 4Department of Neurology, Chonnam National University Medical School, Gwangju, Korea.
- 5Department of Neurosurgery, Chonnam National University Medical School, Gwangju, Korea.
- 6Center for Creative Biomedical Scientists, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2417334
- DOI: http://doi.org/10.3340/jkns.2016.59.6.544
Abstract
OBJECTIVE
Cerebral endothelial cells have unique biological features and are fascinating candidate cells for stroke therapy.
METHODS
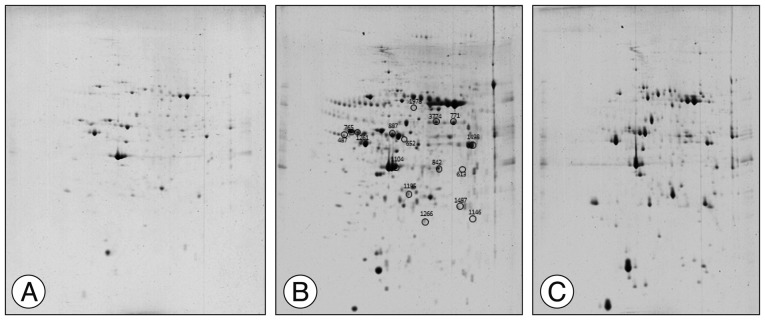
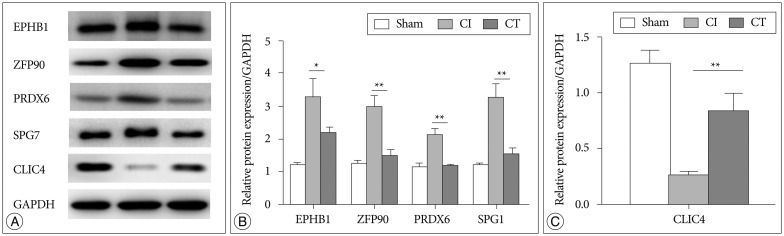
In order to understand the molecular mechanisms of human cerebral endothelial cell (hCMEC/D3) transplantation in a rat stroke model, we performed proteomic analysis using 2-dimensional electrophoresis and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Protein expression was confirmed by quantitative real-time PCR and Western blot.
RESULTS
Several protein spots were identified by gel electrophoresis in the sham, cerebral ischemia (CI), and CI with hCMEC/D3 treatment cerebral ischemia with cell transplantation (CT) groups, and we identified 14 differentially expressed proteins in the CT group. Proteins involved in mitochondrial dysfunction (paraplegin matrix AAA peptidase subunit, SPG7), neuroinflammation (peroxiredoxin 6, PRDX6), and neuronal death (zinc finger protein 90, ZFP90) were markedly reduced in the CT group compared with the CI group. The expression of chloride intracellular channel 4 proteins involved in post-ischemic vasculogenesis was significantly decreased in the CI group but comparable to sham in the CT group.
CONCLUSION
These results contribute to our understanding of the early phase processes that follow cerebral endothelial cell treatment in CI. Moreover, some of the identified proteins may present promising new targets for stroke therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Milk Fat Globule-Epidermal Growth Factor VIII Ameliorates Brain Injury in the Subacute Phase of Cerebral Ischemia in an Animal Model
Jong-Il Choi, Ho-Young Kang, Choongseong Han, Dong-Hun Woo, Jong-Hoon Kim, Dong-Hyuk Park
J Korean Neurosurg Soc. 2020;63(2):163-170. doi: 10.3340/jkns.2019.0188.
Reference
-
1. Bernardi P, Forte M. Commentary : SPG7 is an essential and conserved component of the mitochondrial permeability transition pore. Front Physiol. 2015; 6:320. PMID: 26581158.2. Bohman S, Matsumoto T, Suh K, Dimberg A, Jakobsson L, Yuspa S, et al. Proteomic analysis of vascular endothelial growth factor-induced endothelial cell differentiation reveals a role for chloride intracellular channel 4 (CLIC4) in tubular morphogenesis. J Biol Chem. 2005; 280:42397–42404. PMID: 16239224.
Article3. Brea D, Agulla J, Staes A, Gevaert K, Campos F, Sobrino T, et al. Study of protein expression in peri-infarct tissue after cerebral ischemia. Sci Rep. 2015; 5:12030. PMID: 26153530.4. Calderone A, Jover T, Noh KM, Tanaka H, Yokota H, Lin Y, et al. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci. 2003; 23:2112–2121. PMID: 12657670.
Article5. Chalothorn D, Zhang H, Smith JE, Edwards JC, Faber JE. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ Res. 2009; 105:89–98. PMID: 19478202.
Article6. Chen JH, Kuo HC, Lee KF, Tsai TH. Global proteomic analysis of brain tissues in transient ischemia brain damage in rats. Int J Mol Sci. 2015; 16:11873–11891. PMID: 26016499.
Article7. Choi KH, Kim HS, Park MS, Kim JT, Kim JH, Cho KA, et al. Regulation of caveolin-1 expression determines early brain edema after experimental focal cerebral ischemia. Stroke. 2016; 47:1336–1343. PMID: 27012742.
Article8. Cohen LK, Jensen MB. Scaffolds for intracerebral grafting of neural progenitor cells after cerebral infarction : a systematic review. Arch Neurosci. 2015; 2:e25364. PMID: 26835472.9. Cuadrado E, Rosell A, Colomé N, Hernández-Guillamon M, García-Berrocoso T, Ribo M, et al. The proteome of human brain after ischemic stroke. J Neuropathol Exp Neurol. 2010; 69:1105–1115. PMID: 20940630.
Article10. Granvogl B, Plöscher M, Eichacker LA. Sample preparation by in-gel digestion for mass spectrometry-based proteomics. Anal Bioanal Chem. 2007; 389:991–1002. PMID: 17639354.
Article11. Guo Y, Shi D, Li W, Liang C, Wang H, Ye Z, et al. Effects of cerebral microvascular endothelial cells and vascular endothelial growth factor on the proliferation and differentiation of NSCs : a comparative study. Br J Neurosurg. 2010; 24:62–68. PMID: 20158355.
Article12. Harvey A, Yen TY, Aizman I, Tate C, Case C. Proteomic analysis of the extracellular matrix produced by mesenchymal stromal cells : implications for cell therapy mechanism. PLoS One. 2013; 8:e79283. PMID: 24244468.13. Hata L, Murakami M, Kuwahara K, Nakagawa Y, Kinoshita H, Usami S, et al. Zinc-finger protein 90 negatively regulates neuron-restrictive silencer factor-mediated transcriptional repression of fetal cardiac genes. J Mol Cell Cardiol. 2011; 50:972–981. PMID: 21284946.
Article14. Dongsheng H, Zhuo Z, Jiamin L, Hailan M, Lijuan H, Fan C, et al. Proteomic analysis of the peri-infarct area after human umbilical cord mesenchymal stem cell transplantation in experimental stroke. Aging Dis. 2016; 7:623–634. PMID: 27699085.
Article15. Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013; 44:3473–3481. PMID: 24130140.
Article16. Jackson MJ, Papa S, Bolaños J, Bruckdorfer R, Carlsen H, Elliott RM, et al. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med. 2002; 23:209–285. PMID: 12079772.
Article17. Kalladka D, Muir KW. Brain repair : cell therapy in stroke. Stem Cells Cloning. 2014; 7:31–44. PMID: 24627643.18. Kim JS. Stroke becomes the 3rd important cause of death in Korea; is it a time to toast? J Stroke. 2014; 16:55–56. PMID: 24949308.
Article19. Koussounadis A, Langdon SP, Um IH, Harrison DJ, Smith VA. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci Rep. 2015; 5:10775. PMID: 26053859.
Article20. Kuang X, Wang LF, Yu L, Li YJ, Wang YN, He Q, et al. Ligustilide ameliorates neuroinflammation and brain injury in focal cerebral ischemia/reperfusion rats : involvement of inhibition of TLR4/peroxiredoxin 6 signaling. Free Radic Biol Med. 2014; 71:165–175. PMID: 24681253.
Article21. Kuwahara K, Saito Y, Ogawa E, Takahashi N, Nakagawa Y, Naruse Y, et al. The neuron-restrictive silencer element-neuron-restrictive silencer factor system regulates basal and endothelin 1-inducible atrial natriuretic peptide gene expression in ventricular myocytes. Mol Cell Biol. 2001; 21:2085–2097. PMID: 11238943.
Article22. Li WY, Jin RL, Hu XY, Chen W, Bang OY. Proteomic analysis of ischemic rat brain after human mesenchymal stem cell transplantation. Tissue Eng Regen Med. 2014; 11:333–339.
Article23. Lucitti JL, Tarte NJ, Faber JE. Chloride intracellular channel 4 is required for maturation of the cerebral collateral circulation. Am J Physiol Heart Circ Physiol. 2015; 309:H1141–H1150. PMID: 26276819.
Article24. Maier T, Güell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009; 583:3966–3973. PMID: 19850042.
Article25. Moon JH, Na JY, Lee MC, Choi KH, Lee JK, Min JJ, et al. Neuroprotective effects of systemic cerebral endothelial cell transplantation in a rat model of cerebral ischemia. Am J Transl Res. 2016; 8:2343–2353. PMID: 27347342.26. Noh KM, Hwang JY, Follenzi A, Athanasiadou R, Miyawaki T, Greally JM, et al. Repressor element-1 silencing transcription factor (REST)-dependent epigenetic remodeling is critical to ischemia-induced neuronal death. Proc Natl Acad Sci U S A. 2012; 109:E962–E971. PMID: 22371606.
Article27. Ooi L, Wood IC. Chromatin crosstalk in development and disease : lessons from REST. Nat Rev Genet. 2007; 8:544–554. PMID: 17572692.
Article28. Pandya JD, Sullivan PG, Pettigrew LC. Focal cerebral ischemia and mitochondrial dysfunction in the TNFα-transgenic rat. Brain Res. 2011; 1384:151–160. PMID: 21300036.
Article29. Rodríguez-Frutos B, Otero-Ortega L, Gutiérrez-Fernández M, Fuentes B, Ramos-Cejudo J, Díez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016; 7:378–387. PMID: 27384771.
Article30. Schmidt NO, Koeder D, Messing M, Mueller FJ, Aboody KS, Kim SU, et al. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res. 2009; 1268:24–37. PMID: 19285048.
Article31. Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, et al. SPG7 is an essential and conserved component of the mitochondrial permeability transition pore. Mol Cell. 2015; 60:47–62. PMID: 26387735.
Article32. Shichita T, Hasegawa E, Kimura A, Morita R, Sakaguchi R, Takada I, et al. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat Med. 2012; 18:911–917. PMID: 22610280.
Article33. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke : a phase 1/2a study. Stroke. 2016; 47:1817–1824. PMID: 27256670.
Article34. Ulmasov B, Bruno J, Gordon N, Hartnett ME, Edwards JC. Chloride intracellular channel protein-4 functions in angiogenesis by supporting acidification of vacuoles along the intracellular tubulogenic pathway. Am J Pathol. 2009; 174:1084–1096. PMID: 19197003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Upregulation of miR-20b Protects Against Cerebral Ischemic Stroke by Targeting Thioredoxin Interacting Protein (TXNIP)
- Ischemic brain injury decreases dynamin-like protein 1 expression in a middle cerebral artery occlusion animal model and glutamate-exposed HT22 cells
- Heterologous corneal endothelial cell transplantation: human corneal endothelial cell transplantation in Lewis rats
- Animal Model of Cerebral Ischemia
- Cord blood-derived CD34(+) cells promotes functional recovery in transient middle cerebral artery occlusion model of rat