Endocrinol Metab.
2015 Sep;30(3):235-245. 10.3803/EnM.2015.30.3.235.
Connecting Myokines and Metabolism
- Affiliations
-
- 1Division of Endocrinology, Diabetes and Metabolism, and the Institute for Diabetes, Obesity and Metabolism, Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA. ahima@mail.med.upenn.edu
- 2Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea.
- KMID: 2407072
- DOI: http://doi.org/10.3803/EnM.2015.30.3.235
Abstract
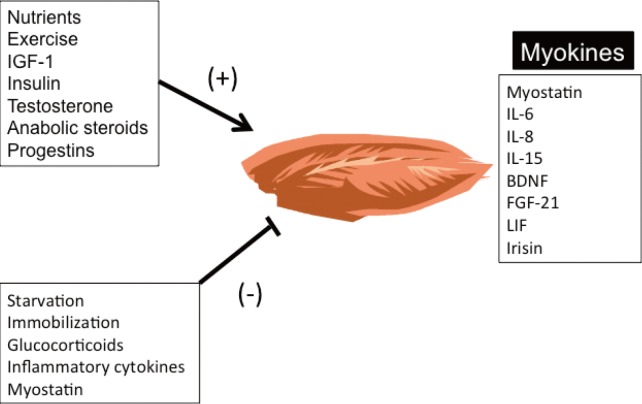
- Skeletal muscle is the largest organ of the body in non-obese individuals and is now considered to be an endocrine organ. Hormones (myokines) secreted by skeletal muscle mediate communications between muscle and liver, adipose tissue, brain, and other organs. Myokines affect muscle mass and myofiber switching, and have profound effects on glucose and lipid metabolism and inflammation, thus contributing to energy homeostasis and the pathogenesis of obesity, diabetes, and other diseases. In this review, we summarize recent findings on the biology of myokines and provide an assessment of their potential as therapeutic targets.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Computed Tomography-Derived Myosteatosis and Metabolic Disorders
Iva Miljkovic, Chantal A. Vella, Matthew Allison
Diabetes Metab J. 2021;45(4):482-491. doi: 10.4093/dmj.2020.0277.Musclin Is Related to Insulin Resistance and Body Composition, but Not to Body Mass Index or Cardiorespiratory Capacity in Adults
Yeliana L. Sánchez, Manuela Yepes-Calderón, Luis Valbuena, Andrés F. Milán, María C. Trillos-Almanza, Sergio Granados, Miguel Peña, Mauricio Estrada-Castrillón, Juan C. Aristizábal, Raúl Narvez-Sanchez, Jaime Gallo-Villegas, Juan C. Calderón
Endocrinol Metab. 2021;36(5):1055-1068. doi: 10.3803/EnM.2021.1104.
Reference
-
1. Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003; 289:323–330. PMID: 12525233.
Article2. Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, et al. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008; 40:1863–1872. PMID: 18845966.
Article3. Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, et al. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011; 6:e19657. PMID: 21647427.
Article4. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–1350. PMID: 11333990.
Article5. Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999; 341:650–658. PMID: 10460816.
Article6. Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008; 15:239–246. PMID: 18525377.
Article7. Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007; 18:137–157. PMID: 17130685.8. Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000; 89:81–88. PMID: 10904038.
Article9. Friedrichsen M, Mortensen B, Pehmoller C, Birk JB, Wojtaszewski JF. Exercise-induced AMPK activity in skeletal muscle: role in glucose uptake and insulin sensitivity. Mol Cell Endocrinol. 2013; 366:204–214. PMID: 22796442.
Article10. Turner N, Cooney GJ, Kraegen EW, Bruce CR. Fatty acid metabolism, energy expenditure and insulin resistance in muscle. J Endocrinol. 2014; 220:T61–T79. PMID: 24323910.
Article11. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013; 280:4294–4314. PMID: 23517348.
Article12. Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr. 2014; 99:237S–242S. PMID: 24284445.
Article13. Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol. 2014; 49:59–68. PMID: 24237131.
Article14. Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010; 91:1128S–1132S. PMID: 20181807.
Article15. Schakman O, Kalista S, Barbe C, Loumaye A, Thissen JP. Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol. 2013; 45:2163–2172. PMID: 23806868.
Article16. Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol. 2013; 45:2200–2208. PMID: 23800384.
Article17. Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol. 2013; 45:2215–2229. PMID: 23770121.
Article18. Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the National Health and Nutrition Examination Survey III. PLoS One. 2010; 5:e10805. PMID: 22421977.
Article19. Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obes Res Clin Pract. 2013; 7:e301–e307. PMID: 24306159.
Article20. Moon SS. Low skeletal muscle mass is associated with insulin resistance, diabetes, and metabolic syndrome in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009-2010. Endocr J. 2014; 61:61–70. PMID: 24088600.
Article21. Han K, Park YM, Kwon HS, Ko SH, Lee SH, Yim HW, et al. Sarcopenia as a determinant of blood pressure in older Koreans: findings from the Korea National Health and Nutrition Examination Surveys (KNHANES) 2008-2010. PLoS One. 2014; 9:e86902. PMID: 24489804.
Article22. Goldstein MS. Humoral nature of the hypoglycemic factor of muscular work. Diabetes. 1961; 10:232–234. PMID: 13706674.
Article23. Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, et al. Searching for the exercise factor: is IL-6 a candidate? J Muscle Res Cell Motil. 2003; 24:113–119. PMID: 14609022.24. Bortoluzzi S, Scannapieco P, Cestaro A, Danieli GA, Schiaffino S. Computational reconstruction of the human skeletal muscle secretome. Proteins. 2006; 62:776–792. PMID: 16342272.
Article25. Henningsen J, Rigbolt KT, Blagoev B, Pedersen BK, Kratchmarova I. Dynamics of the skeletal muscle secretome during myoblast differentiation. Mol Cell Proteomics. 2010; 9:2482–2496. PMID: 20631206.
Article26. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012; 8:457–465. PMID: 22473333.
Article27. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997; 387:83–90. PMID: 9139826.28. Szabo G, Dallmann G, Muller G, Patthy L, Soller M, Varga L. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome. 1998; 9:671–672. PMID: 9680391.29. Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006; 38:813–818. PMID: 16751773.
Article30. Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997; 17:71–74. PMID: 9288100.
Article31. Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997; 7:910–916. PMID: 9314496.32. Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004; 350:2682–2688. PMID: 15215484.
Article33. Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008; 29:513–534. PMID: 18591260.
Article34. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006; 124:471–484. PMID: 16469695.
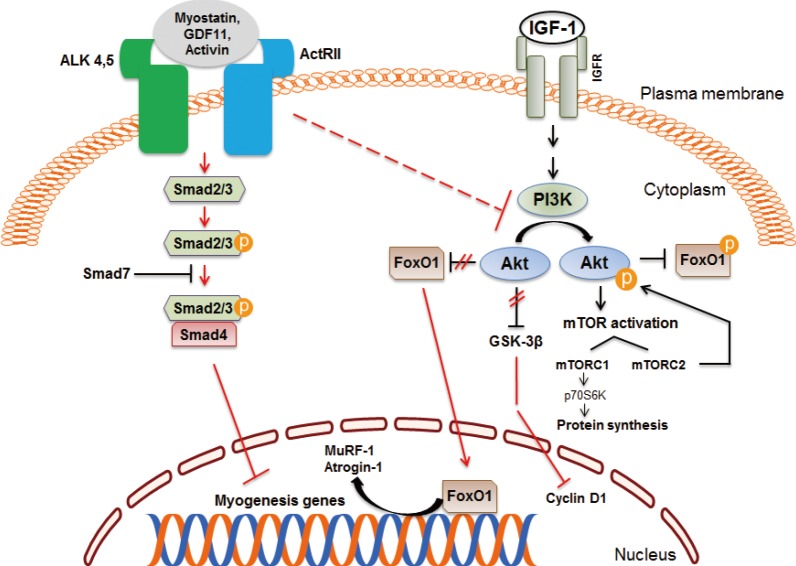
Article35. Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem. 2007; 282:3799–3808. PMID: 17130121.36. Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, et al. Down-regulation of Akt/mammalian target of rapamycin signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinology. 2009; 150:286–294. PMID: 18801898.
Article37. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009; 296:C1258–C1270. PMID: 19357233.
Article38. Derynck R, Zhang Y, Feng XH. Smads: transcriptional activators of TGF-beta responses. Cell. 1998; 95:737–740. PMID: 9865691.39. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001; 98:9306–9311. PMID: 11459935.
Article40. Zhu X, Topouzis S, Liang LF, Stotish RL. Myostatin signaling through Smad2, Smad3 and Smad4 is regulated by the inhibitory Smad7 by a negative feedback mechanism. Cytokine. 2004; 26:262–272. PMID: 15183844.
Article41. Forbes D, Jackman M, Bishop A, Thomas M, Kambadur R, Sharma M. Myostatin auto-regulates its expression by feedback loop through Smad7 dependent mechanism. J Cell Physiol. 2006; 206:264–272. PMID: 16110474.
Article42. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004; 117:399–412. PMID: 15109499.
Article43. Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004; 14:395–403. PMID: 15125842.
Article44. Rodriguez J, Vernus B, Chelh I, Cassar-Malek I, Gabillard JC, Hadj Sassi A, et al. Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell Mol Life Sci. 2014; 71:4361–4371. PMID: 25080109.
Article45. Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun. 2002; 291:701–706. PMID: 11855847.
Article46. McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002; 109:595–601. PMID: 11877467.
Article47. Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009; 4:e4937. PMID: 19295913.
Article48. Akpan I, Goncalves MD, Dhir R, Yin X, Pistilli EE, Bogdanovich S, et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int J Obes (Lond). 2009; 33:1265–1273. PMID: 19668253.
Article49. Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001; 15:2203–2208. PMID: 11544177.
Article50. Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003; 112:197–208. PMID: 12843127.51. Goncalves MD, Pistilli EE, Balduzzi A, Birnbaum MJ, Lachey J, Khurana TS, et al. Akt deficiency attenuates muscle size and function but not the response to ActRIIB inhibition. PLoS One. 2010; 5:e12707. PMID: 20856813.
Article52. Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, et al. A novel therapeutic approach to treating obesity through modulation of TGFbeta signaling. Endocrinology. 2012; 153:3133–3146. PMID: 22549226.53. Zhang C, McFarlane C, Lokireddy S, Masuda S, Ge X, Gluckman PD, et al. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia. 2012; 55:183–193. PMID: 21927895.
Article54. Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J. 2013; 27:1981–1989. PMID: 23362117.55. Choi SJ, Yablonka-Reuveni Z, Kaiyala KJ, Ogimoto K, Schwartz MW, Wisse BE. Increased energy expenditure and leptin sensitivity account for low fat mass in myostatin-deficient mice. Am J Physiol Endocrinol Metab. 2011; 300:E1031–E1037. PMID: 21427410.
Article56. Attie KM, Borgstein NG, Yang Y, Condon CH, Wilson DM, Pearsall AE, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle Nerve. 2013; 47:416–423. PMID: 23169607.
Article57. Padhi D, Higano CS, Shore ND, Sieber P, Rasmussen E, Smith MR. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2014; 99:E1967–E1975. PMID: 24971661.
Article58. Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes. 2009; 58:30–38. PMID: 18835929.
Article59. Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc. 2011; 43:1828–1835. PMID: 21364474.
Article60. Bartoccioni E, Michaelis D, Hohlfeld R. Constitutive and cytokine-induced production of interleukin-6 by human myoblasts. Immunol Lett. 1994; 42:135–138. PMID: 7890313.
Article61. Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998; 508(Pt 3):949–953. PMID: 9518745.
Article62. Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, et al. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001; 15:2748–2750. PMID: 11687509.
Article63. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008; 88:1379–1406. PMID: 18923185.
Article64. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013; 17:162–184. PMID: 23395166.
Article65. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2004; 52:1098–1104. PMID: 15209647.
Article66. Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia. 2006; 49:2078–2085. PMID: 16791618.
Article67. Hamer M, Sabia S, Batty GD, Shipley MJ, Tabak AG, Singh-Manoux A, et al. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation. 2012; 126:928–933. PMID: 22891048.68. Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK. Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004; 287:E1189–E1194. PMID: 15304377.
Article69. Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK. Effect of exercise, training, and glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol (1985). 2005; 99:2075–2079. PMID: 16099893.
Article70. Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006; 55:2688–2697. PMID: 17003332.71. van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, et al. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003; 88:3005–3010. PMID: 12843134.
Article72. Bruce CR, Dyck DJ. Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab. 2004; 287:E616–E621. PMID: 15149950.73. Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006; 20:3364–3375. PMID: 16945991.
Article74. Steensberg A, Fischer CP, Sacchetti M, Keller C, Osada T, Schjerling P, et al. Acute interleukin-6 administration does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans. J Physiol. 2003; 548(Pt 2):631–638. PMID: 12640021.
Article75. Wolsk E, Mygind H, Grondahl TS, Pedersen BK, van Hall G. IL-6 selectively stimulates fat metabolism in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010; 299:E832–E840. PMID: 20823453.
Article76. Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003; 285:E433–E437. PMID: 12857678.
Article77. Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science. 1994; 264:965–968. PMID: 8178155.78. Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995; 14:3654–3663. PMID: 7641685.
Article79. Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994; 13:2822–2830. PMID: 8026467.
Article80. Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995; 136:3669–3672. PMID: 7628408.
Article81. Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM. Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res. 2002; 280:55–63. PMID: 12372339.82. Furmanczyk PS, Quinn LS. Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biol Int. 2003; 27:845–851. PMID: 14499665.
Article83. Pistilli EE, Alway SE. Systemic elevation of interleukin-15 in vivo promotes apoptosis in skeletal muscles of young adult and aged rats. Biochem Biophys Res Commun. 2008; 373:20–24. PMID: 18555009.84. He Y, Wu X, Khan RS, Kastin AJ, Cornelissen-Guillaume GG, Hsuchou H, et al. IL-15 receptor deletion results in circadian changes of locomotor and metabolic activity. J Mol Neurosci. 2010; 41:315–321. PMID: 20012227.
Article85. Wu X, He Y, Hsuchou H, Kastin AJ, Rood JC, Pan W. Essential role of interleukin-15 receptor in normal anxiety behavior. Brain Behav Immun. 2010; 24:1340–1346. PMID: 20600810.
Article86. Pistilli EE, Devaney JM, Gordish-Dressman H, Bradbury MK, Seip RL, Thompson PD, et al. Interleukin-15 and interleukin-15R alpha SNPs and associations with muscle, bone, and predictors of the metabolic syndrome. Cytokine. 2008; 43:45–53. PMID: 18514540.87. Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol (1985). 2004; 97:2214–2219. PMID: 15531573.
Article88. Di Renzo L, Bigioni M, Bottini FG, Del Gobbo V, Premrov MG, Cianci R, et al. Normal Weight Obese syndrome: role of single nucleotide polymorphism of IL-1 5Ralpha and MTHFR 677C-->T genes in the relationship between body composition and resting metabolic rate. Eur Rev Med Pharmacol Sci. 2006; 10:235–245. PMID: 17121316.89. Nielsen AR, Hojman P, Erikstrup C, Fischer CP, Plomgaard P, Mounier R, et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J Clin Endocrinol Metab. 2008; 93:4486–4493. PMID: 18697873.
Article90. Di Renzo L, Gloria-Bottini F, Saccucci P, Bigioni M, Abenavoli L, Gasbarrini G, et al. Role of interleukin-15 receptor alpha polymorphisms in normal weight obese syndrome. Int J Immunopathol Pharmacol. 2009; 22:105–113. PMID: 19309557.91. Pistilli EE, Bogdanovich S, Garton F, Yang N, Gulbin JP, Conner JD, et al. Loss of IL-15 receptor alpha alters the endurance, fatigability, and metabolic characteristics of mouse fast skeletal muscles. J Clin Invest. 2011; 121:3120–3132. PMID: 21765213.92. O'Connell GC, Pistilli EE. Interleukin-15 directly stimulates pro-oxidative gene expression in skeletal muscle in-vitro via a mechanism that requires interleukin-15 receptor alpha. Biochem Biophys Res Commun. 2015; 458:614–619. PMID: 25681766.93. O'Connell G, Guo G, Stricker J, Quinn LS, Ma A, Pistilli EE. Muscle-specific deletion of exons 2 and 3 of the IL-15RA gene in mice: effects on contractile properties of fast and slow muscles. J Appl Physiol (1985). 2015; 118:437–448. PMID: 25505029.94. Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002; 16:1879–1886. PMID: 12468452.
Article95. Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004; 18:357–368. PMID: 15004004.
Article96. Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006; 27:728–735. PMID: 17018837.97. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–468. PMID: 22237023.98. Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, et al. Evidence against a beneficial effect of irisin in humans. PLoS One. 2013; 8:e73680. PMID: 24040023.
Article99. Erickson HP. Irisin and FNDC5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte. 2013; 2:289–293. PMID: 24052909.100. Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013; 98:E769–E778. PMID: 23436919.
Article101. Hecksteden A, Wegmann M, Steffen A, Kraushaar J, Morsch A, Ruppenthal S, et al. Irisin and exercise training in humans: results from a randomized controlled training trial. BMC Med. 2013; 11:235. PMID: 24191966.
Article102. Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014; 281:739–749. PMID: 24237962.103. Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, et al. FNDC5 and irisin in humans: I. predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012; 61:1725–1738. PMID: 23018146.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Muscles on Bone Metabolism—with a Focus on Myokines
- Myokines and interorgan crosstalk: bridging exercise to health promotion and disease prevention
- The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis
- Body Composition, Sarcopenia, and Serum Myokines in Acromegaly: A Narrative Review
- A combination of myokines and genistein suppresses cancer stemness in MCF-7 human breast cancer cells