Korean J Physiol Pharmacol.
2018 Jan;22(1):23-33. 10.4196/kjpp.2018.22.1.23.
Histone deacetylase inhibition attenuates hepatic steatosis in rats with experimental Cushing's syndrome
- Affiliations
-
- 1Department of Pharmacology, Kyungpook National University School of Medicine, Daegu 41944, Korea. inkim@knu.ac.kr
- 2Cardiovascular Research Institute, Kyungpook National University School of Medicine, Daegu 41944, Korea.
- 3Cell and Matrix Research Institute, Kyungpook National University School of Medicine, Daegu 41944, Korea.
- 4BK21 Plus KNU Biomedical Convergence Program, Department of Biomedical Science, Kyungpook National University School of Medicine, Daegu 41944, Korea.
- KMID: 2398552
- DOI: http://doi.org/10.4196/kjpp.2018.22.1.23
Abstract
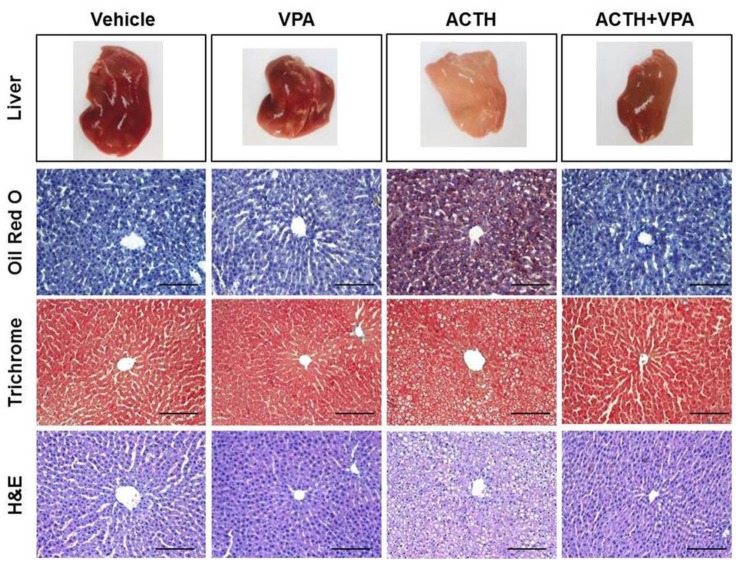
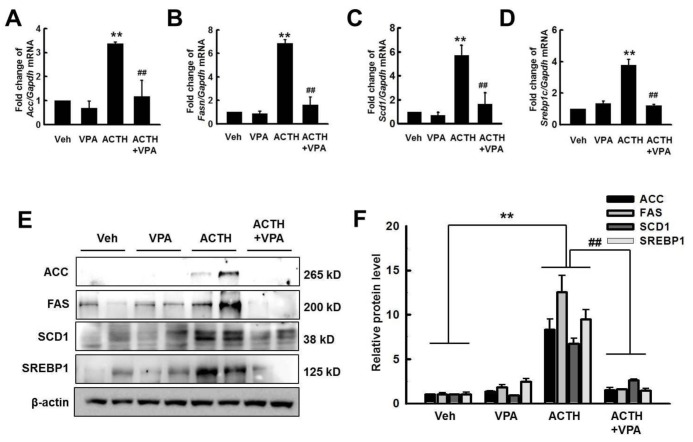
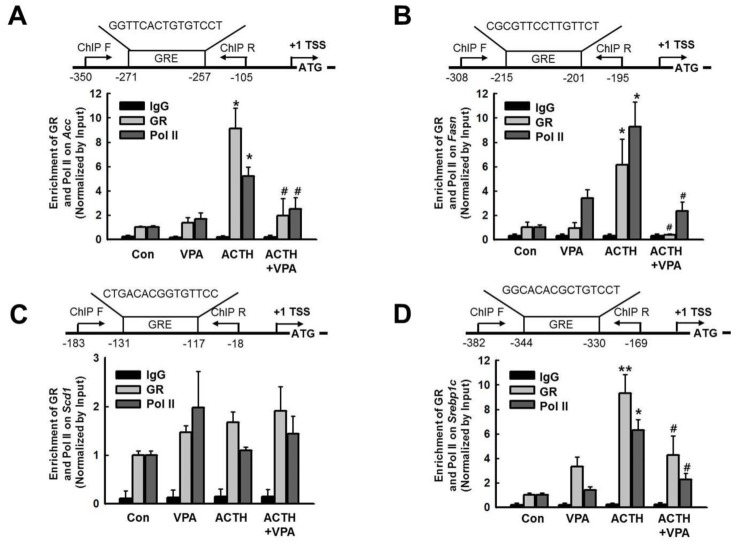
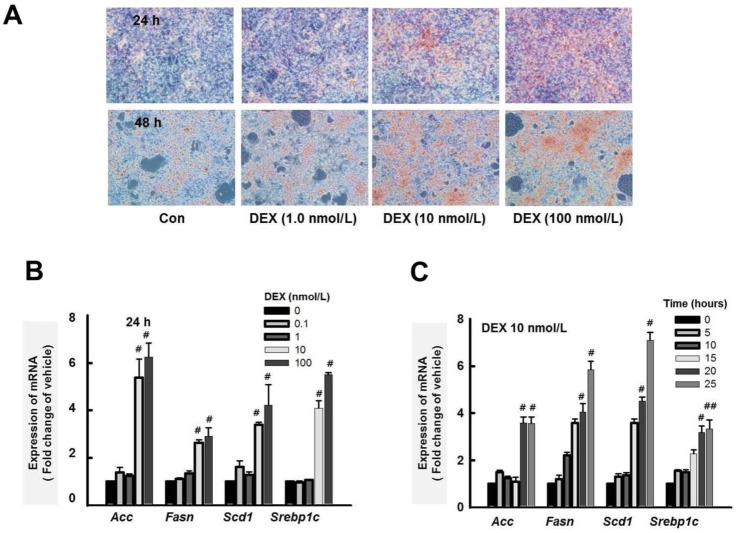
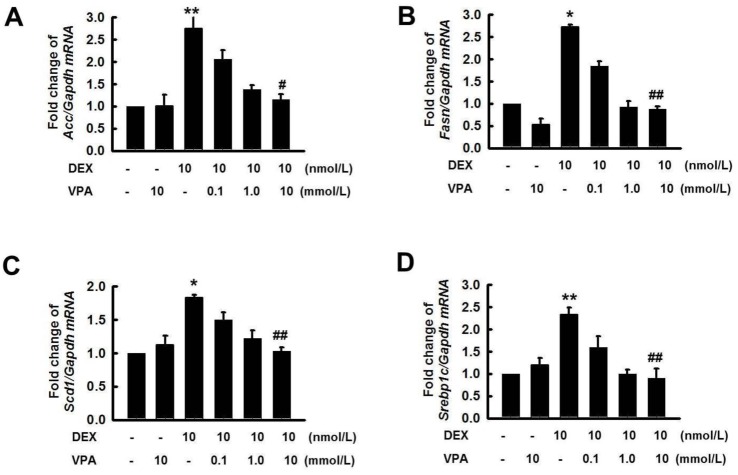
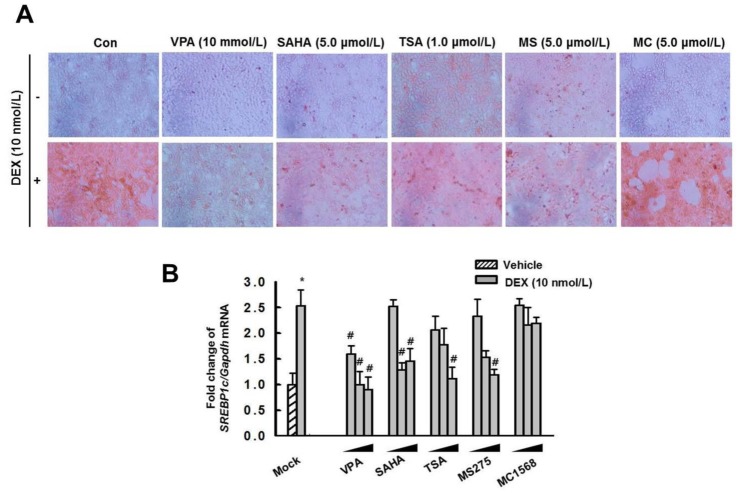
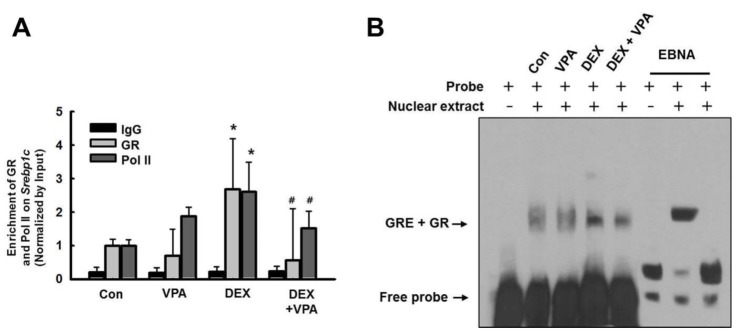
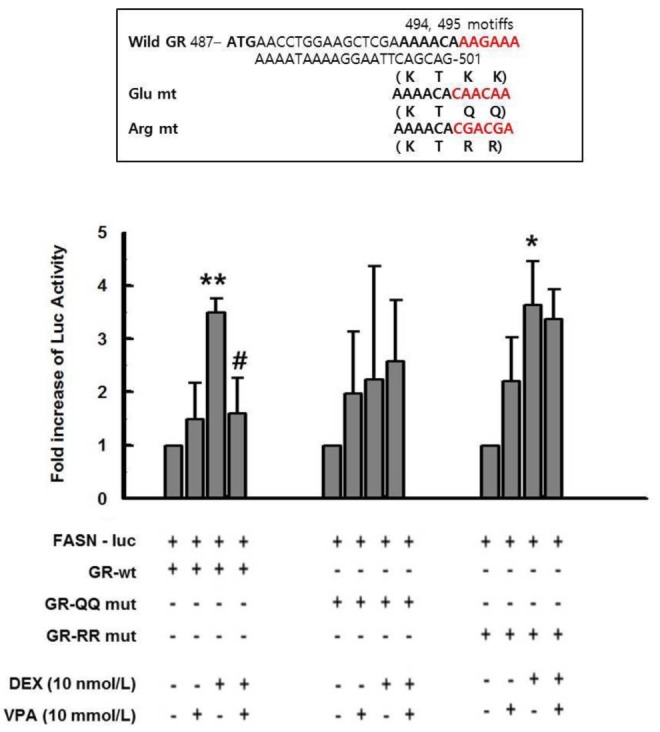
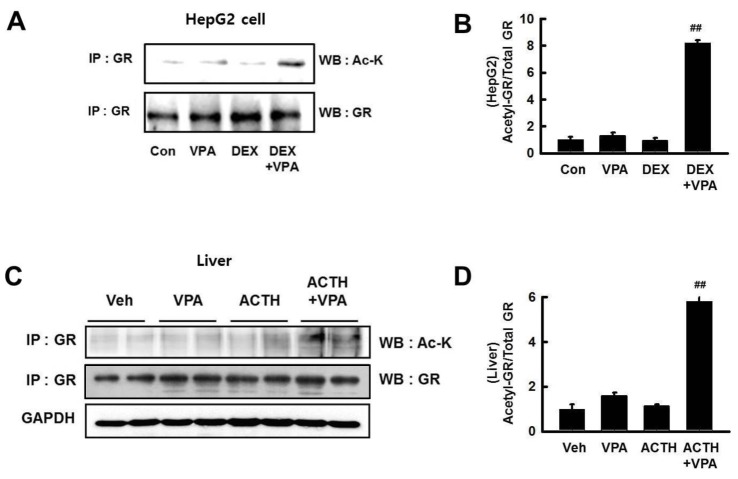
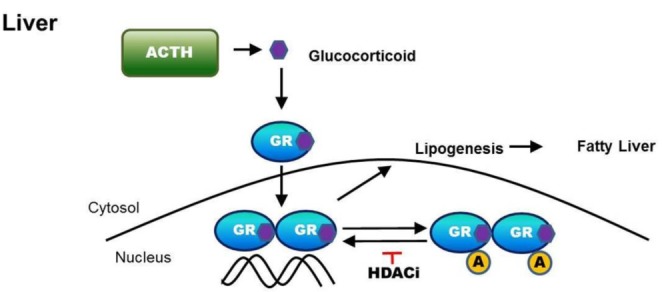
- Cushing's syndrome (CS) is a collection of symptoms caused by prolonged exposure to excess cortisol. Chronically elevated glucocorticoid (GC) levels contribute to hepatic steatosis. We hypothesized that histone deacetylase inhibitors (HDACi) could attenuate hepatic steatosis through glucocorticoid receptor (GR) acetylation in experimental CS. To induce CS, we administered adrenocorticotropic hormone (ACTH; 40 ng/kg/day) to Sprague-Dawley rats by subcutaneous infusion with osmotic mini-pumps. We administered the HDACi, sodium valproate (VPA; 0.71% w/v), in the drinking water. Treatment with the HDACi decreased steatosis and the expression of lipogenic genes in the livers of CS rats. The enrichment of GR at the promoters of the lipogenic genes, such as acetyl-CoA carboxylase (Acc), fatty acid synthase (Fasn), and sterol regulatory element binding protein 1c (Srebp1c), was markedly decreased by VPA. Pan-HDACi and an HDAC class I-specific inhibitor, but not an HDAC class II a-specific inhibitor, attenuated dexamethasone (DEX)-induced lipogenesis in HepG2 cells. The transcriptional activity of Fasn was decreased by pretreatment with VPA. In addition, pretreatment with VPA decreased DEX-induced binding of GR to the glucocorticoid response element (GRE). Treatment with VPA increased the acetylation of GR in ACTH-infused rats and DEX-induced HepG2 cells. Taken together, these results indicate that HDAC inhibition attenuates hepatic steatosis hrough GR acetylation in experimental CS.
Keyword
MeSH Terms
-
Acetyl-CoA Carboxylase
Acetylation
Adrenocorticotropic Hormone
Animals
Cushing Syndrome*
Dexamethasone
Drinking Water
Hep G2 Cells
Histone Deacetylase Inhibitors
Histone Deacetylases*
Histones*
Hydrocortisone
Infusions, Subcutaneous
Lipogenesis
Liver
Rats*
Rats, Sprague-Dawley
Receptors, Glucocorticoid
Response Elements
Sterol Regulatory Element Binding Protein 1
Valproic Acid
Acetyl-CoA Carboxylase
Adrenocorticotropic Hormone
Dexamethasone
Drinking Water
Histone Deacetylase Inhibitors
Histone Deacetylases
Histones
Hydrocortisone
Receptors, Glucocorticoid
Sterol Regulatory Element Binding Protein 1
Valproic Acid
Figure
Cited by 1 articles
-
Maternal high-fructose intake during pregnancy and lactation induces metabolic syndrome in adult offspring
Soohyeon Koo, Mina Kim, Hyun Min Cho, Inkyeom Kim
Nutr Res Pract. 2021;15(2):160-172. doi: 10.4162/nrp.2021.15.2.160.
Reference
-
1. Lonser RR, Nieman L, Oldfield EH. Cushing's disease: pathobiology, diagnosis, and management. J Neurosurg. 2017; 126:404–417. PMID: 27104844.
Article2. Woods CP, Hazlehurst JM, Tomlinson JW. Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol. 2015; 154:94–103. PMID: 26241028.
Article3. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995; 332:1351–1362. PMID: 7715646.
Article4. Rosen J, Miner JN. The search for safer glucocorticoid receptor ligands. Endocr Rev. 2005; 26:452–464. PMID: 15814846.
Article5. Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. Hepatic steatosis in Cushing's syndrome: a radiological assessment using computed tomography. Eur J Endocrinol. 2003; 149:543–548. PMID: 14640995.
Article6. Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008; 28:339–350. PMID: 18956290.
Article7. Lewis JR, Mohanty SR. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci. 2010; 55:560–578. PMID: 20101463.
Article8. Kassel O, Herrlich P. Crosstalk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007; 275:13–29. PMID: 17689856.
Article9. Wang JC, Gray NE, Kuo T, Harris CA. Regulation of triglyceride metabolism by glucocorticoid receptor. Cell Biosci. 2012; 2:19. PMID: 22640645.
Article10. Lemke U, Krones-Herzig A, Berriel Diaz M, Narvekar P, Ziegler A, Vegiopoulos A, Cato AC, Bohl S, Klingmüller U, Screaton RA, Müller-Decker K, Kersten S, Herzig S. The glucocorticoid receptor controls hepatic dyslipidemia through Hes1. Cell Metab. 2008; 8:212–223. PMID: 18762022.
Article11. Mueller KM, Kornfeld JW, Friedbichler K, Blaas L, Egger G, Esterbauer H, Hasselblatt P, Schlederer M, Haindl S, Wagner KU, Engblom D, Haemmerle G, Kratky D, Sexl V, Kenner L, Kozlov AV, Terracciano L, Zechner R, Schuetz G, Casanova E, Pospisilik JA, Heim MH, Moriggl R. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011; 54:1398–1409. PMID: 21725989.
Article12. Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006; 60:520–528. PMID: 16949786.13. Galliher-Beckley AJ, Williams JG, Cidlowski JA. Ligand-independent phosphorylation of the glucocorticoid receptor integrates cellular stress pathways with nuclear receptor signaling. Mol Cell Biol. 2011; 31:4663–4675. PMID: 21930780.
Article14. Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005; 2005:pe48. PMID: 16204701.
Article15. Patel BM, Raghunathan S, Porwal U. Cardioprotective effects of magnesium valproate in type 2 diabetes mellitus. Eur J Pharmacol. 2014; 728:128–134. PMID: 24530414.
Article16. Khan S, Kumar S, Jena G. Valproic acid reduces insulin-resistance, fat deposition and FOXO1-mediated gluconeogenesis in type-2 diabetic rat. Biochimie. 2016; 125:42–52. PMID: 26944797.
Article17. Buchwald M, Krämer OH, Heinzel T. HDACi.targets beyond chromatin. Cancer Lett. 2009; 280:160–167. PMID: 19342155.18. Kadiyala V, Patrick NM, Mathieu W, Jaime-Frias R, Pookhao N, An L, Smith CL. Class I lysine deacetylases facilitate glucocorticoid-induced transcription. J Biol Chem. 2013; 288:28900–28912. PMID: 23946490.
Article19. Lauffer BE, Mintzer R, Fong R, Mukund S, Tam C, Zilberleyb I, Flicke B, Ritscher A, Fedorowicz G, Vallero R, Ortwine DF, Gunzner J, Modrusan Z, Neumann L, Koth CM, Lupardus PJ, Kaminker JS, Heise CE, Steiner P. Histone deacetylase (HDAC) inhibitor kinetic rate constants correlate with cellular histone acetylation but not transcription and cell viability. J Biol Chem. 2013; 288:26926–26943. PMID: 23897821.
Article20. Kang SH, Seok YM, Song MJ, Lee HA, Kurz T, Kim I. Histone deacetylase inhibition attenuates cardiac hypertrophy and fibrosis through acetylation of mineralocorticoid receptor in spontaneously hypertensive rats. Mol Pharmacol. 2015; 87:782–791. PMID: 25667225.
Article21. Lee HA, Lee DY, Cho HM, Kim SY, Iwasaki Y, Kim IK. Histone deacetylase inhibition attenuates transcriptional activity of mineralocorticoid receptor through its acetylation and prevents development of hypertension. Circ Res. 2013; 112:1004–1012. PMID: 23421989.
Article22. Lee HA, Song MJ, Seok YM, Kang SH, Kim SY, Kim I. Histone deacetylase 3 and 4 complex stimulates the transcriptional activity of the mineralocorticoid receptor. PLoS One. 2015; 10:e0136801. PMID: 26305553.
Article23. Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci. 2013; 34:518–530. PMID: 23953592.
Article24. Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J Endocrinol. 2008; 197:189–204. PMID: 18434349.
Article25. Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing's syndrome. Neuroendocrinology. 2010; 92(Suppl 1):86–90. PMID: 20829625.
Article26. Lagace DC, Nachtigal MW. Inhibition of histone deacetylase activity by valproic acid blocks adipogenesis. J Biol Chem. 2004; 279:18851–18860. PMID: 14985358.
Article27. Catalioto RM, Maggi CA, Giuliani S. Chemically distinct HDAC inhibitors prevent adipose conversion of subcutaneous human white preadipocytes at an early stage of the differentiation program. Exp Cell Res. 2009; 315:3267–3280. PMID: 19766628.
Article28. Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009; 50(Suppl):S138–S143. PMID: 19047759.
Article29. Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009; 100:1369–1372. PMID: 19352381.
Article30. Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010; 45:199–214. PMID: 20218765.31. Peserico A, Simone C. Physical and functional HAT/HDAC interplay regulates protein acetylation balance. J Biomed Biotechnol. 2011; 2011:371832. PMID: 21151613.
Article32. Nader N, Chrousos GP, Kino T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J. 2009; 23:1572–1583. PMID: 19141540.
Article33. Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. J Exp Med. 2006; 203:7–13. PMID: 16380507.
Article34. Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics. 2012; 4:5. PMID: 22414492.
Article35. Chittiboina P, Lu J, Wang X, Piazza MG, Zhuang Z. Histone deacetylase inhibitor vorinostat is a novel, promising treatment for cushing disease. Neurosurgery. 2016; 63(Suppl 1):183.36. Sul HS, Wang D. Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu Rev Nutr. 1998; 18:331–351. PMID: 9706228.
Article37. Zhao LF, Iwasaki Y, Zhe W, Nishiyama M, Taguchi T, Tsugita M, Kambayashi M, Hashimoto K, Terada Y. Hormonal regulation of acetyl-CoA carboxylase isoenzyme gene transcription. Endocr J. 2010; 57:317–324. PMID: 20139635.
Article38. Reddy TE, Pauli F, Sprouse RO, Neff NF, Newberry KM, Garabedian MJ, Myers RM. Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 2009; 19:2163–2171. PMID: 19801529.
Article39. So AY, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007; 3:e94. PMID: 17559307.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases
- Sequence-Dependent Radiosensitization of Histone Deacetylase Inhibitors Trichostatin A and SK-7041
- Hepatic Fibrosis and Steatosis in Metabolic Syndrome
- Activation of ATM-dependent DNA Damage Signal Pathway by a Histone Deacetylase Inhibitor, Trichostatin A
- A Case of Cushing's Syndrome