Cancer Res Treat.
2007 Sep;39(3):125-130.
Activation of ATM-dependent DNA Damage Signal Pathway by a Histone Deacetylase Inhibitor, Trichostatin A
- Affiliations
-
- 1Department of Biological Sciences, College of Natural Sciences and Department of Molecular Science and Technology, Ajou University, Suwon, Korea. jsjlee@mail.ajou.ac.kr
Abstract
-
PURPOSE: Ataxia-telangiectasia mutated (ATM) kinase regulates diverse cellular DNA damage responses, including genome surveillance, cell growth, and gene expression. While the role of histone acetylation/deacetylation in gene expression is well established, little is known as to whether this modification can activate an ATM-dependent signal pathway, and whether this modification can thereby be implicated in an ATM-mediated DNA damage response.
MATERIALS AND METHODS
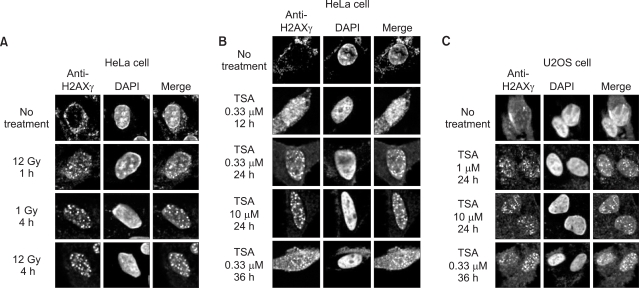
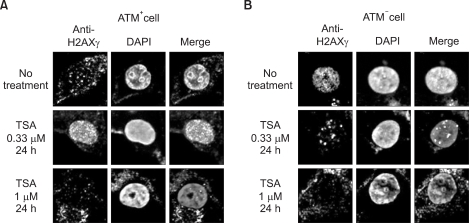
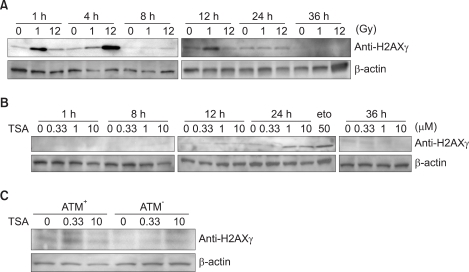
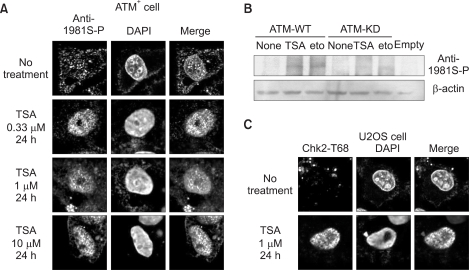
Formation of H2AXgamma foci was examined in HeLa and U2OS cells following treatment with a histone deacetylase inhibitor, Trichostatin A (TSA). We determine an ATM-dependency of the TSA-induced DNA damage signal pathway using isogenic A-T (ATM square) and control (ATM+) cells. We monitored the phosphorylation of ATM, an ATM-downstream effector kinase, Chk2, and H2AXgamma to detect the activation of the ATM-dependent DNA damage signal pathway.
RESULTS
Exposure of cells to TSA results in the formation of H2AXgamma foci in HeLa and U2OS cells. The TSA-induced formation of H2AXgamma foci occurs in an ATM-dependent manner. TSA induces phosphorylation of serine 1981 of ATM, accumulation of phosphorylated H2AX and Chk2, and formation of H2AX foci, in a manner analogous to genotoxic DNA damage.
CONCLUSION
In this work, we show that TSA induces a DNA damage signaling pathway in an ATM-dependent manner. These results suggest that ATM can respond to altered histone acetylation induced by the histone deacetylase inhibitor, TSA.
Keyword
MeSH Terms
Figure
Reference
-
1. Ju R, Muller MT. Histone deacetylase inhibitors activate p21(WAF1) expression via ATM. Cancer Res. 2003; 63:2891–2897. PMID: 12782595.2. Gatei M, Young D, Cerosaletti KM, Desai-Mehta A, Spring K, Kozlov S, et al. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nat Genet. 2000; 25:115–119. PMID: 10802669.
Article3. Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000; 1:179–186. PMID: 11252893.
Article4. Maya R, Balass M, Kim ST, Shkedy D, Leal JF, Shifman O, et al. ATM-depedent phosphoryaltion of Mdm2 on serine 395: role in p53 activation by DNA damage. Genes Dev. 2001; 15:1067–1077. PMID: 11331603.5. Welcsh PL, Lee MK, Gonzalez-Hernandez RM, Black DJ, Mahadevappa M, Swisher EM, et al. BRCa1 transcriptionally regulates genes involved in breast tumorigenesis. Proc Natl Acad Sci USA. 2002; 99:7560–7565. PMID: 12032322.
Article6. Pernin D, Uhrhammer N, Verrelle P, Bignon YJ, Bay JO. p53 activation by PI-3K family kinases after DNA double-strand breaks. Bull Cancer. 2000; 87:635–641. PMID: 11038413.7. Sluss HK, Armata H, Gallant J, Jones SN. Phosphorylation of serine 18 regulates p53 functions in mice. Mol Cell Biol. 2004; 24:976–984. PMID: 14729946.8. Waterman MJ, Stavridi ES, Waterman JL, Halazonetis TD. ATM-dependent activation of p53 involves dephosphorylation and association with 14-3-3 proteins. Nat Genet. 1998; 19:175–178. PMID: 9620776.
Article9. Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998; 18:4377–4384. PMID: 9632821.10. Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002; 108:475–487. PMID: 11909519.
Article11. Qiu P, Li L. Histone acetylation and recruitment of serum responsive factor and CREB-binding protein onto SM22 promoter during SM22 gene expression. Circ Res. 2002; 90:858–865. PMID: 11988486.
Article12. Brush MH, Guardiola A, Connor JH, Yao TP, Shenolikar S. Deacetylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J Biol Chem. 2004; 279:7685–7691. PMID: 14670976.13. Gelmetti V, Zhang J, Fanelli M, Minucci S, Pelicci PG, Lazar MA. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol Cell Biol. 1998; 18:7185–7191. PMID: 9819405.
Article14. Hirose T, Sowa Y, Takahashi S, Saito S, Yasuda C, Shindo N, et al. p53-independent induction of Gadd45 by histone deacetylase inhibitor: coordinate regulation by transcription factors Oct-1 and NF-Y. Oncogene. 2003; 22:7762–7773. PMID: 14586402.
Article15. Zeng L, Zhang Y, Chien S, Liu X, Shyy JY. The role of p53 deacetylation in p21Waf1 regulation by laminar flow. J Biol Chem. 2003; 278:24594–24599. PMID: 12716906.16. Noh EJ, Lee JS. Functional interplay between modulation of histone deacetylase activity and its regulatory role in G2-M transition. Biochem Biophys Res Commun. 2003; 310:267–273. PMID: 14521905.
Article17. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003; 421:499–506. PMID: 12556884.
Article18. Vergani L, Fugazza G, Chessa L, Nicolini C. Changes of chromatin condensation in one patient with ataxia telangiectasia disorder: a structural study. J Cell Biochem. 1999; 75:578–586. PMID: 10572241.
Article19. Kim GD, Choi YH, Dimtchev A, Jeong SJ, Dritschilo A, Jung M. Sensing of ionizaing radiation-induced DNA damage by ATM through interaction with histone deacetylase. J Biol Chem. 1999; 274:31127–31130. PMID: 10531300.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Functional Link between DNA Damage Responses and Transcriptional Regulation by ATM in Response to a Histone Deacetylase Inhibitor TSA
- ATM modulates transcription in response to histone deacetylase inhibition as part of its DNA damage response
- Apoptotic Effect of Combination Treatment with a Proteasome Inhibitor, Lactacystin, and a Histone Deacetylase Inhibitor, Trichostatin A, on MCF-7 Cells
- Role of histone deacetylase activity in the developing lateral line neuromast of zebrafish larvae
- Improved Therapeutic Effect against Leukemia by a Combination of the Histone Methyltransferase Inhibitor Chaetocin and the Histone Deacetylase Inhibitor Trichostatin A