Asia Pac Allergy.
2014 Oct;4(4):212-221. 10.5415/apallergy.2014.4.4.212.
Innate lymphoid cells and cytokines of the novel subtypes of helper T cells in asthma
- Affiliations
-
- 1Acquired Immunodeficiency Research Center, Isfahan University of Medical Sciences, Isfahan 81746-73461, Iran.
- 2Immunology Department, Isfahan University of Medical Sciences, Isfahan 81746-73461, Iran.
- 3Cellular and Molecular Immunology Research Center, Isfahan University of Medical Sciences, Isfahan 81746-73461, Iran. mghakemi@med.mui.ac.ir
- 4Department of Biostatistics and Epidemiology, School of Health, Isfahan University of Medical Sciences, Isfahan 81746-73461, Iran.
- KMID: 2397102
- DOI: http://doi.org/10.5415/apallergy.2014.4.4.212
Abstract
- BACKGROUND
In this study, the expression of interleukin-9 (IL-9), IL-17, IL-22, and IL-25 genes that might be the potential predisposing factors for asthma as well as count of innate lymphoid cells (ILCs) as another source of inflammatory cytokines have been evaluated.
OBJECTIVE
The aim of this study was to evaluate the expression of newly identified helper T cells signature cytokines and amount of ILCs.
METHODS
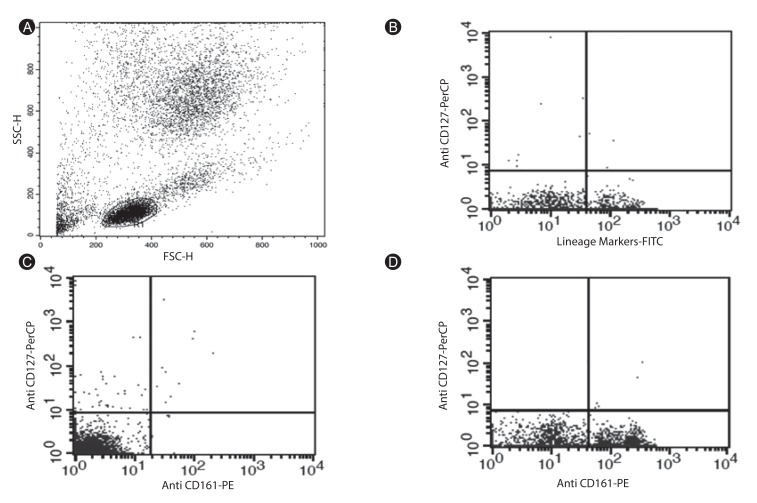
Blood and sputum samples from 23 patients with moderate to severe asthma and 23 healthy volunteers were collected. The types of allergens to which our patients were sensitive were defined using immunoblotting method. Gene expression of studied cytokines was evaluated using quantitative transcription-polymerase chain reaction and ILCs were counted by the flow cytometry method.
RESULTS
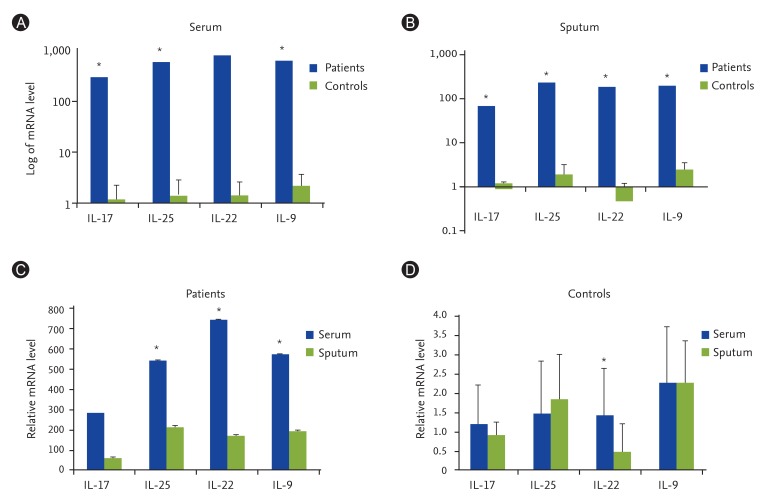
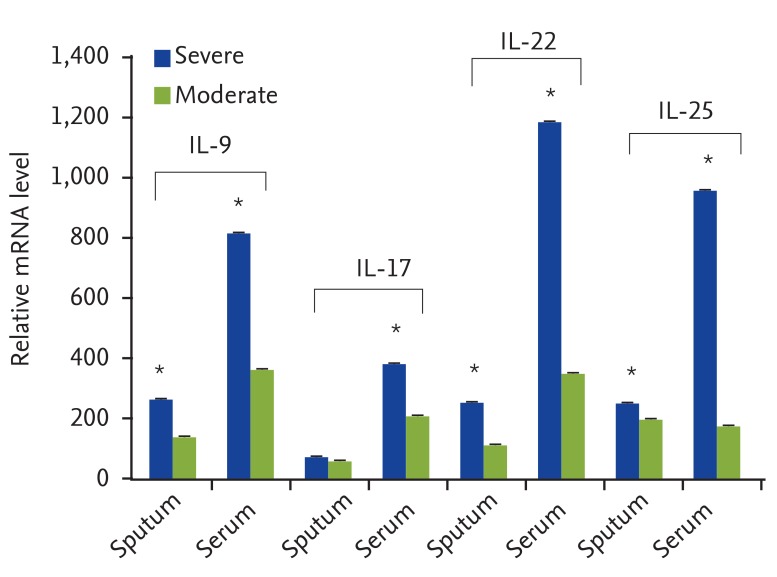
In this research, the gene expressions of IL-9, IL-17, IL-22, and IL-25 were significantly higher in asthmatics, especially in the severe form of the disease. This increase was even higher in serum samples compared with sputum samples. Counting ILCs revealed their increase in comparison with normal people.
CONCLUSION
We showed the importance of IL-25, IL-22, IL-17, and IL-9 cytokines in patients with asthma as their expression levels are increased and these increase are correlated with the severity of the disease. We also showed that the increased amount of ILCs in asthmatics could confirm their potential role in the immunopathogenesis of asthma as another source of inflammatory cytokines.
MeSH Terms
Figure
Cited by 2 articles
-
In the memory of Professor Felicidad Cua-Lim
Yoon-Seok Chang
Asia Pac Allergy. 2014;4(4):185-186. doi: 10.5415/apallergy.2014.4.4.185.Anti-Interleukin-9 Antibody Increases the Effect of Allergen-Specific Immunotherapy in Murine Allergic Rhinitis
Ji-Hyeon Shin, Do Hyun Kim, Boo-Young Kim, Sung Won Kim, Se Hwan Hwang, Joohyung Lee, Soo Whan Kim
Allergy Asthma Immunol Res. 2017;9(3):237-246. doi: 10.4168/aair.2017.9.3.237.
Reference
-
2. Walker HK, Halt WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. Chapter 37. Gong H. Wheezing and asthma [Internet]. 3rd ed. Boston: Butterworths;1990. cited 2014 Feb 15. Available from: http://www.ncbi.nlm.nih.gov/books/NBK358.3. Cookson W. The alliance of genes and environment in asthma and allergy. Nature. 1999; 402(6760 Suppl):B5–B11. PMID: 10586889.
Article4. Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, Holt PG. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998; 160:4730–4737. PMID: 9590218.5. Haley KJ, Sunday ME, Wiggs BR, Kozakewich HP, Reilly JJ, Mentzer SJ, Sugarbaker DJ, Doerschuk CM, Drazen JM. Inflammatory cell distribution within and along asthmatic airways. Am J Respir Crit Care Med. 1998; 158:565–572. PMID: 9700136.
Article6. Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med. 1996; 154:1505–1510. PMID: 8912772.
Article8. Smith H. Asthma, inflammation, eosinophils and bronchial hyperresponsiveness. Clin Exp Allergy. 1992; 22:187–197. PMID: 1571812.
Article9. Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999; 160:1001–1008. PMID: 10471631.
Article10. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986; 136:2348–2357. PMID: 2419430.11. Lindén A, Hoshino H, Laan M. Airway neutrophils and interleukin-17. Eur Respir J. 2000; 15:973–977. PMID: 10853869.
Article12. Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, Jabeen R, McKinley C, Ahyi AN, Han L, Nguyen ET, Robertson MJ, Perumal NB, Tepper RS, Nutt SL, Kaplan MH. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010; 11:527–534. PMID: 20431622.
Article13. Louahed J, Kermouni A, Van Snick J, Renauld JC. IL-9 induces expression of granzymes and high-affinity IgE receptor in murine T helper clones. J Immunol. 1995; 154:5061–5070. PMID: 7730612.14. Dugas B, Renauld JC, Pene J, Bonnefoy JY, Peti-Frère C, Braquet P, Bousquet J, Van Snick J, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol. 1993; 23:1687–1692. PMID: 7686859.
Article15. Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R. Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res. 2014; 3:127. PMID: 24949298.
Article16. Adibrad M, Deyhimi P, Ganjalikhani Hakemi M, Behfarnia P, Shahabuei M, Rafiee L. Signs of the presence of Th17 cells in chronic periodontal disease. J Periodontal Res. 2012; 47:525–531. PMID: 22309127.
Article17. Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007; 13:139–145. PMID: 17290272.
Article18. Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006; 16:902–907. PMID: 17088898.
Article19. Rocha B. Comment on "Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells". Science. 2005; 308:1553. PMID: 15947157.20. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004; 21:241–254. PMID: 15308104.
Article21. Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007; 8:942–949. PMID: 17676045.22. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest. 2009; 119:3573–3585. PMID: 19920355.
Article23. Hirose K, Takahashi K, Nakajima H. Roles of IL-22 in allergic airway inflammation. J Allergy (Cairo). 2013; 2013:260518. PMID: 23577040.
Article24. Vock C, Hauber HP, Wegmann M. The other T helper cells in asthma pathogenesis. J Allergy (Cairo). 2010; 2010:519298. PMID: 20976014.
Article25. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007; 204:1509–1517. PMID: 17562814.
Article26. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007; 120:1324–1331. PMID: 17889290.
Article27. Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011; 12:1055–1062. PMID: 21909091.
Article28. Klein Wolterink RG, Hendriks RW. Type 2 innate lymphocytes in allergic airway inflammation. Curr Allergy Asthma Rep. 2013; 13:271–280. PMID: 23563812.
Article29. Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005; 172:149–160. PMID: 15849323.
Article30. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D'Agostino R Jr, Castro M, Curran-Everett D, Fitzpatrick AM, Gaston B, Jarjour NN, Sorkness R, Calhoun WJ, Chung KF, Comhair SA, Dweik RA, Israel E, Peters SP, Busse WW, Erzurum SC, Bleecker ER. National Heart, Lung, and Blood Institute's Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–323. PMID: 19892860.
Article31. March ME, Sleiman PM, Hakonarson H. The genetics of asthma and allergic disorders. Discov Med. 2011; 11:35–45. PMID: 21276409.32. Baraldo S, Faffe DS, Moore PE, Whitehead T, McKenna M, Silverman ES, Panettieri RA Jr, Shore SA. Interleukin-9 influences chemokine release in airway smooth muscle: role of ERK. Am J Physiol Lung Cell Mol Physiol. 2003; 284:L1093–L1102. PMID: 12588703.
Article33. Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, Humbles AA. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011; 183:865–875. PMID: 20971830.
Article34. Goswami R, Kaplan MH. A brief history of IL-9. J Immunol. 2011; 186:3283–3288. PMID: 21368237.
Article35. Toda M, Tulic MK, Levitt RC, Hamid Q. A calcium-activated chloride channel (HCLCA1) is strongly related to IL-9 expression and mucus production in bronchial epithelium of patients with asthma. J Allergy Clin Immunol. 2002; 109:246–250. PMID: 11842292.
Article36. Abdelilah S, Latifa K, Esra N, Cameron L, Bouchaib L, Nicolaides N, Levitt R, Hamid Q. Functional expression of IL-9 receptor by human neutrophils from asthmatic donors: role in IL-8 release. J Immunol. 2001; 166:2768–2774. PMID: 11160343.
Article37. Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010; 207:2479–2491. PMID: 20921287.
Article38. Ganjalikhani Hakemi M, Ghaedi K, Andalib A, Hosseini M, Rezaei A. Optimization of human Th17 cell differentiation in vitro: evaluating different polarizing factors. In Vitro Cell Dev Biol Anim. 2011; 47:581–592. PMID: 21853398.
Article39. Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006; 7:135. PMID: 17083726.
Article40. Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001; 108:430–438. PMID: 11544464.
Article41. Alyasin S, Karimi MH, Amin R, Babaei M, Darougar S. Interleukin-17 gene expression and serum levels in children with severe asthma. Iran J Immunol. 2013; 10:177–185. PMID: 24076595.42. Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002; 168:5397–5402. PMID: 12023331.
Article43. Takahashi K, Hirose K, Kawashima S, Niwa Y, Wakashin H, Iwata A, Tokoyoda K, Renauld JC, Iwamoto I, Nakayama T, Nakajima H. IL-22 attenuates IL-25 production by lung epithelial cells and inhibits antigen-induced eosinophilic airway inflammation. J Allergy Clin Immunol. 2011; 128:1067–1076.e1-6. PMID: 21794904.
Article44. Farfariello V, Amantini C, Nabissi M, Morelli MB, Aperio C, Caprodossi S, Carlucci A, Bianchi AM, Santoni G. IL-22 mRNA in peripheral blood mononuclear cells from allergic rhinitic and asthmatic pediatric patients. Pediatr Allergy Immunol. 2011; 22:419–423. PMID: 21535180.
Article45. Tsuji M, Kawamoto T, Koriyama C, Matsumura F. IL-22 mRNA expression in blood samples as a useful biomarker for assessing the adverse health effects of PCBs on allergic children. Int J Environ Res Public Health. 2012; 9:4321–4332. PMID: 23330224.
Article46. Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol. 2010; 151:297–307. PMID: 19844129.
Article47. Schnyder B, Lima C, Schnyder-Candrian S. Interleukin-22 is a negative regulator of the allergic response. Cytokine. 2010; 50:220–227. PMID: 20194033.
Article48. Afran L. Asthma and allergy: IL-25-responsive myeloid cells promote type 2 lung pathology. Nat Rev Immunol. 2012; 12:doi: 10.1038/nri3239.49. Gregory LG, Jones CP, Walker SA, Sawant D, Gowers KH, Campbell GA, McKenzie AN, Lloyd CM. IL-25 drives remodelling in allergic airways disease induced by house dust mite. Thorax. 2013; 68:82–90. PMID: 23093652.
Article50. Liu F, Wu JX, Zhao JP, Li HJ, Liu W, Bi WX, Dong L. IL-25 derived from epithelial cells has the potential to promote airway remodeling in asthma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012; 28:633–636. PMID: 22691357.