Nutr Res Pract.
2017 Dec;11(6):470-478. 10.4162/nrp.2017.11.6.470.
Anti-fibrotic effects of Orostachys japonicus A. Berger (Crassulaceae) on hepatic stellate cells and thioacetamide-induced fibrosis in rats
- Affiliations
-
- 1Department of Biotechnology, College of Biomedical and Health Sciences, Konkuk University, 268 Chungwon-daero, Chungju-si, Chungbuk 27478, Korea. minds@kku.ac.kr
- 2Department of Applied Life Science, Graduate School of Konkuk University, 268 Chungwon-daero, Chungju-si, Chungbuk 27478, Korea.
- 3Food One Corp., 260 Sincheoksandan 5-ro, Deoksan-myeon, Jincheon-gun, Chungbuk 27850, Korea.
- 4Department of Internal Medicine, School of Medicine, Konkuk University, Chungju, Chungbuk 27376, Korea.
- 5R&D center, Korean Drug Co., Ltd., Seoul 06300, Korea.
- KMID: 2395301
- DOI: http://doi.org/10.4162/nrp.2017.11.6.470
Abstract
- BACKGROUND
/OBJECTIVE: Orostachys japonicus A. Berger (Crassulaceae) has been used in traditional herbal medicines in Korea and other Asian countries to treat various diseases, including liver disorders. In the present study, the anti-fibrotic effects of O. japonicus extract (OJE) in cellular and experimental hepatofibrotic rat models were investigated.
MATERIALS/METHODS
An in vitro hepatic stellate cells (HSCs) system was used to estimate cell viability, cell cycle and apoptosis by MTT assay, flow cytometry, and Annexin V-FITC/PI staining techniques, respectively. In addition, thioacetamide (TAA)-induced liver fibrosis was established in Sprague Dawley rats. Briefly, animals were divided into five groups (n = 8): Control, TAA, OJE 10 (TAA with OJE 10 mg/kg), OJE 100 (TAA with OJE 100 mg/kg) and silymarin (TAA with Silymarin 50 mg/kg). Fibrosis was induced by treatment with TAA (200 mg/kg, i.p.) twice per week for 13 weeks, while OJE and silymarin were administered orally two times per week from week 7 to 13. The fibrotic related gene expression serum biomarkers glutathione and hydroxyproline were estimated by RT-PCR and spectrophotometry, respectively, using commercial kits.
RESULTS
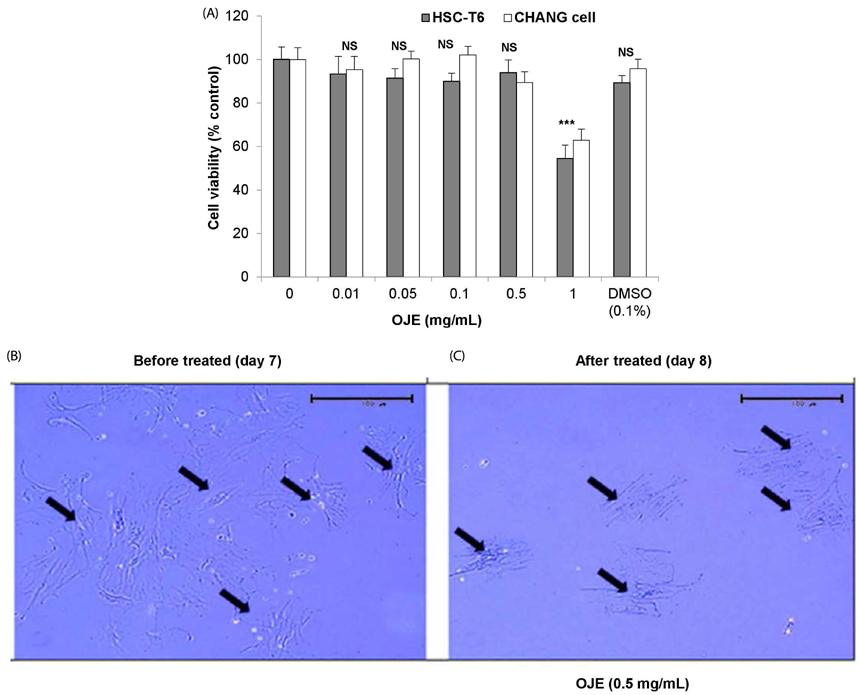
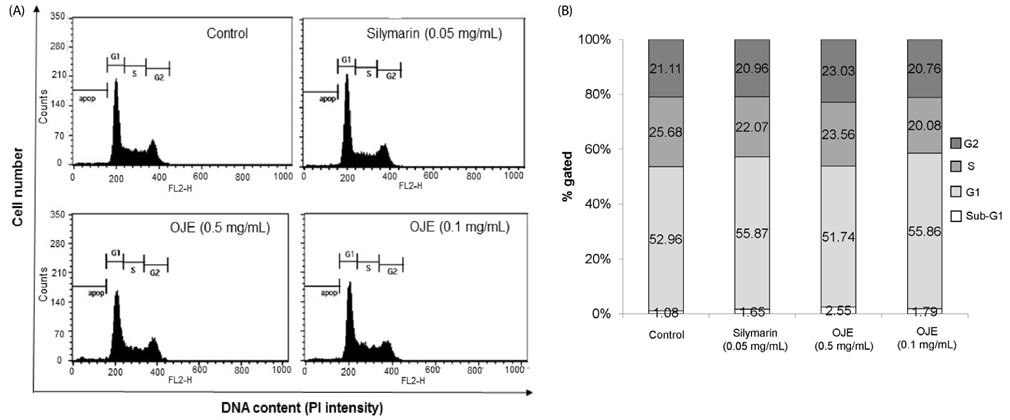
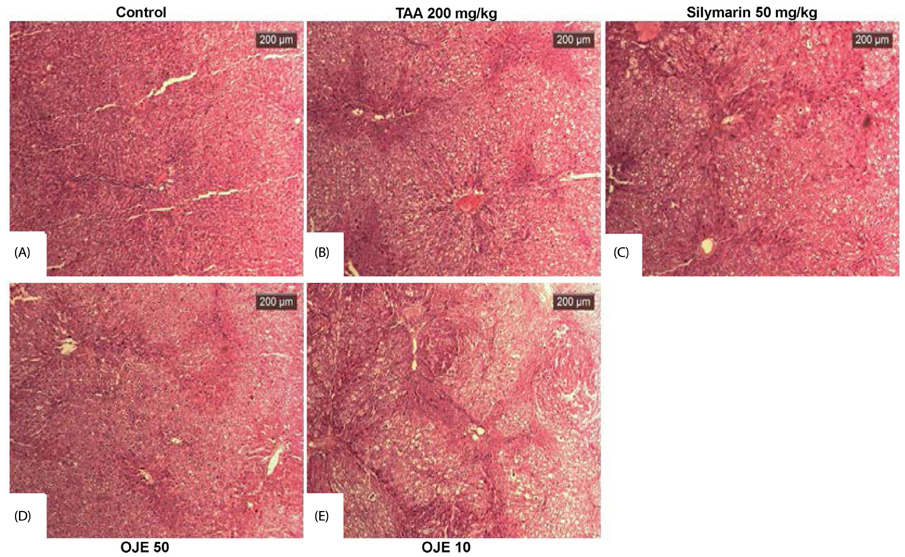
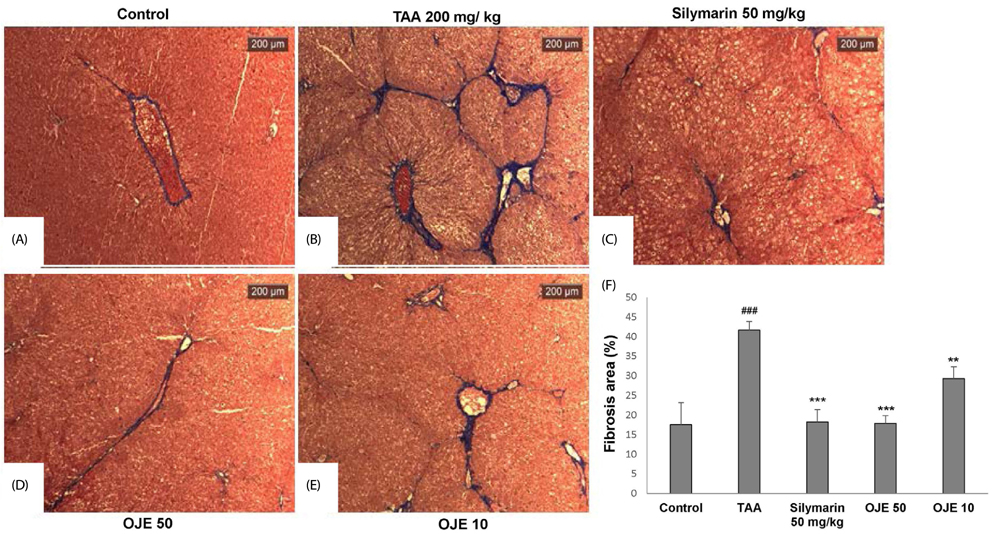
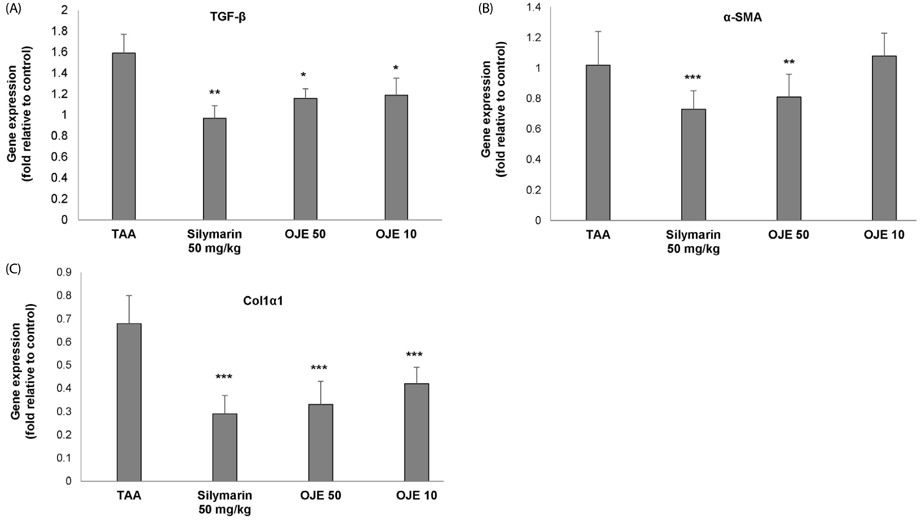
OJE (0.5 and 0.1 mg/mL) and silymarin (0.05 mg/mL) treatment significantly (P < 0.01 and P < 0.001) induced apoptosis (16.95% and 27.48% for OJE and 25.87% for silymarin, respectively) in HSC-T6 cells when compared with the control group (9.09%). Further, rat primary HSCs showed changes in morphology in response to OJE 0.1 mg/mL treatment. In in vivo studies, OJE (10 and 100 mg/kg) treatment significantly ameliorated TAA-induced alterations in levels of serum biomarkers, fibrotic related gene expression, glutathione, and hydroxyproline (P < 0.05-P < 0.001) and rescued the histopathological changes.
CONCLUSIONS
OJE can be developed as a potential agent for the treatment of hepatofibrosis.
Keyword
MeSH Terms
-
Animals
Apoptosis
Asian Continental Ancestry Group
Biomarkers
Cell Cycle
Cell Survival
Fibrosis*
Flow Cytometry
Gene Expression
Glutathione
Hepatic Stellate Cells*
Humans
Hydroxyproline
In Vitro Techniques
Korea
Liver
Liver Cirrhosis
Models, Animal
Rats*
Rats, Sprague-Dawley
Silymarin
Spectrophotometry
Thioacetamide
Biomarkers
Glutathione
Hydroxyproline
Silymarin
Thioacetamide
Figure
Reference
-
1. Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014; 7:193–203.
Article2. Popper H, Uenfriend S. Hepatic fibrosis. Correlation of biochemical and morphologic investigations. Am J Med. 1970; 49:707–721.3. Friedman SL, Maher JJ, Bissell DM. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. Hepatology. 2000; 32:1403–1408.
Article4. Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000; 287:1253–1258.
Article5. Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001; 21:311–335.
Article6. Lee JJ, Yang SY, Kim DH, Hur SJ, Lee JD, Yum MJ, Song MD. Liver fibrosis protective effect of Hovenia dulcis fruit. Curr Top Nutraceutical Res. 2014; 12:43–50.7. Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008; 88:125–172.
Article8. Lee SJ, Shin JH, Kang JR, Hwang CR, Sung NJ. In vitro evaluation of biological activities of Wa-song (Orostachys japonicus A. Berger) and Korean traditional plants mixture. J Korean Soc Food Sci Nutr. 2012; 41:295–301.
Article9. Kim JK. Illustrated Natural Drugs. Seoul: Namsandang Publishing Co;1984.10. Lee SJ, Seo JK, Shin JH, Lee HJ, Sung NJ. Antioxidant activity of Wa-song (Orostachys japonicus A. Berger) according to drying methods. J Korean Soc Food Sci Nutr. 2008; 37:605–611.
Article11. Yoon JA, Son YS. Effects of Opuntia ficus-indica complexes B (OCB) on blood glucose and lipid metabolism in streptozotocin-induced diabetic rats. Korean J Food Nutr. 2009; 22:48–56.12. Lee SJ, Zhang GF, Sung NJ. Hypolipidemic and hypoglycemic effects of Orostachys japonicus A. Berger extracts in streptozotocin-induced diabetic rats. Nutr Res Pract. 2011; 5:301–307.
Article13. Hendriks HF, Verhoofstad WA, Brouwer A, de Leeuw AM, Knook DL. Perisinusoidal fat-storing cells are the main vitamin A storage sites in rat liver. Exp Cell Res. 1985; 160:138–149.
Article14. Knook DL, Seffelaar AM, de Leeuw AM. Fat-storing cells of the rat liver. Their isolation and purification. Exp Cell Res. 1982; 139:468–471.
Article15. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82:70–77.
Article16. Takayama T, Fujita K, Suzuki K, Sakaguchi M, Fujie M, Nagai E, Watanabe S, Ichiyama A, Ogawa Y. Control of oxalate formation from L-hydroxyproline in liver mitochondria. J Am Soc Nephrol. 2003; 14:939–946.
Article17. Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998; 292:447–452.
Article18. Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004; 1:98–105.
Article19. Johnston DE. Special considerations in interpreting liver function tests. Am Fam Physician. 1999; 59:2223–2230.20. McClatchey KD. Clinical Laboratory Medicine. 2nd ed. Philadelphia (PA): Lippincott Wiliams & Wilkins;2002.21. Bruck R, Aeed H, Avni Y, Shirin H, Matas Z, Shahmurov M, Avinoach I, Zozulya G, Weizman N, Hochman A. Melatonin inhibits nuclear factor kappa B activation and oxidative stress and protects against thioacetamide induced liver damage in rats. J Hepatol. 2004; 40:86–93.
Article22. Krane SM. The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids. 2008; 35:703–710.
Article23. Pálfi VK, Perczel A. How stable is a collagen triple helix? An ab initio study on various collagen and beta-sheet forming sequences. J Comput Chem. 2008; 29:1374–1386.
Article24. Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007; 13:3056–3062.25. Liu Y, Wang Z, Kwong SQ, Lui EL, Friedman SL, Li FR, Lam RW, Zhang GC, Zhang H, Ye T. Inhibition of PDGF, TGF-beta, and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol. 2011; 55:612–625.
Article26. Jakowlew SB, Mead JE, Danielpour D, Wu J, Roberts AB, Fausto N. Transforming growth factor-beta (TGF-beta) isoforms in rat liver regeneration: messenger RNA expression and activation of latent TGF-beta. Cell Regul. 1991; 2:535–548.
Article27. Hori N, Okanoue T, Sawa Y, Mori T, Kashima K. Hemodynamic characterization in experimental liver cirrhosis induced by thioacetamide administration. Dig Dis Sci. 1993; 38:2195–2202.
Article28. Park HJ, Young HS, Park KY, Rhee SH, Chung HY, Choi JS. Flavonoids from the whole plants of Orostachys japonicus. Arch Pharm Res. 1991; 14:167–171.29. Yoon Y, Kim KS, Hong SG, Kang BJ, Lee MY, Cho DW. Protective effects of Orostachys japonicus A. Berger (Crassulaceae) on H2O2-induced apoptosis in GT1-1 mouse hypothalamic neuronal cell line. J Ethnopharmacol. 2000; 69:73–78.
Article30. Yoon NY, Min BS, Lee HK, Park JC, Choi JS. A potent anti-complementary acylated sterol glucoside from Orostachys japonicus. Arch Pharm Res. 2005; 28:892–896.
Article31. Hernández-Ortega LD, Alcántar-Díaz BE, Ruiz-Corro LA, Sandoval-Rodriguez A, Bueno-Topete M, Armendariz-Borunda J, Salazar-Montes AM. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J Gastroenterol Hepatol. 2012; 27:1865–1872.
Article32. Zhou YP, Zhang SL, Cheng D, Li HR, Tang ZM, Xue J, Cai W, Dong JH, Zhao L. Preliminary exploration on anti-fibrosis effect of Kaempferol in mice with Schistosoma Japonicum infection. Eur J Inflamm. 2013; 11:161–168.
Article33. Hsieh SC, Wu CH, Wu CC, Yen JH, Liu MC, Hsueh CM, Hsu SL. Gallic acid selectively induces the necrosis of activated hepatic stellate cells via a calcium-dependent calpain I activation pathway. Life Sci. 2014; 102:55–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Orostachys japonicus induce caspase-dependent apoptosis in HeLa human cervical cancer cells
- Hypolipidemic and hypoglycemic effects of Orostachys japonicus A. Berger extracts in streptozotocin-induced diabetic rats
- Role of cytoglobin, a novel radical scavenger, in stellate cell activation and hepatic fibrosis
- Effect of Pentoxifylline on Liver Fibrosis and Cell Cycle Related Proteins in Thioacetamide-Induced Rat Cirrhosis
- Parthenolide-induced apoptosis of hepatic stellate cells and anti-fibrotic effects in an in vivo rat model