Korean J Physiol Pharmacol.
2017 Sep;21(5):531-546. 10.4196/kjpp.2017.21.5.531.
Time-dependent proteomic and genomic alterations in Toll-like receptor-4-activated human chondrocytes: increased expression of lamin A/C and annexins
- Affiliations
-
- 1National Research Laboratory for Mitochondrial Signaling, Department of Physiology, Department of Health Sciences and Technology, BK21 Plus Project Team, College of Medicine, Cardiovascular and Metabolic Disease Center, Inje University, Busan 47392, Korea. phyhanj@inje.ac.kr
- 2Department of Health Technology Development, Health Project Management Team, Korea Health Industry Development Institute (KHIDI), Cheongju 28159, Korea.
- KMID: 2388698
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.531
Abstract
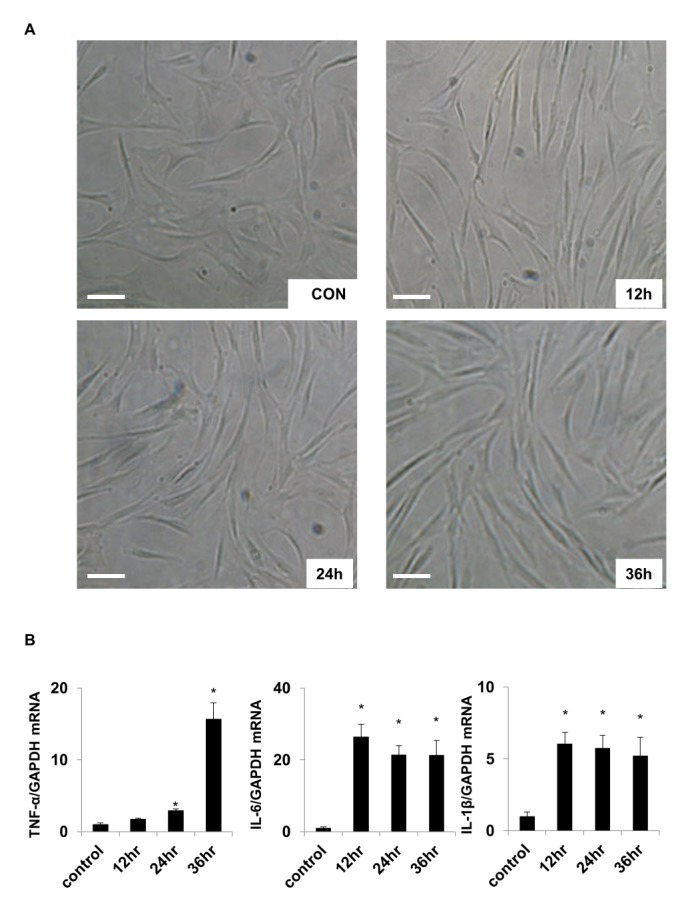
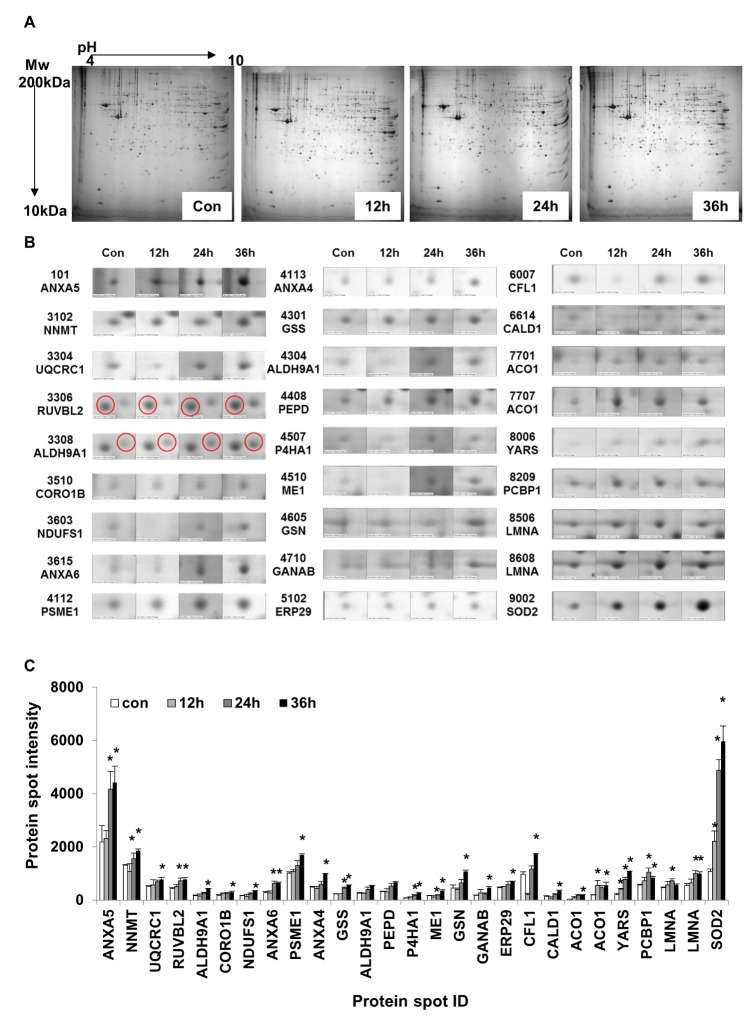
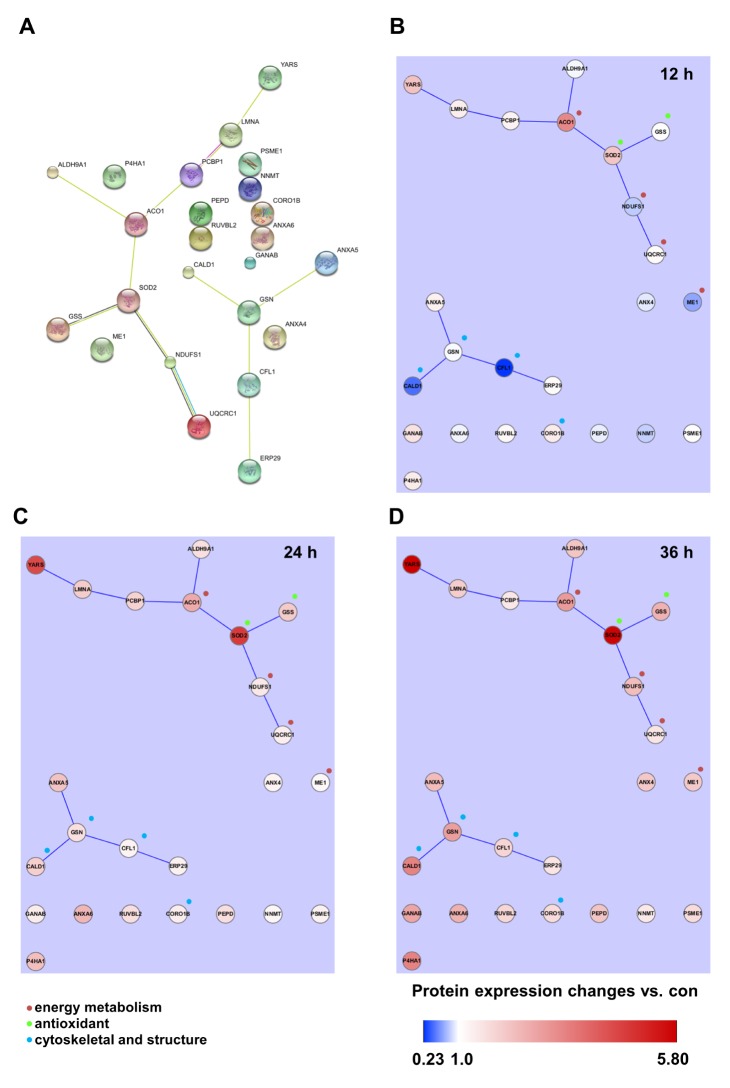
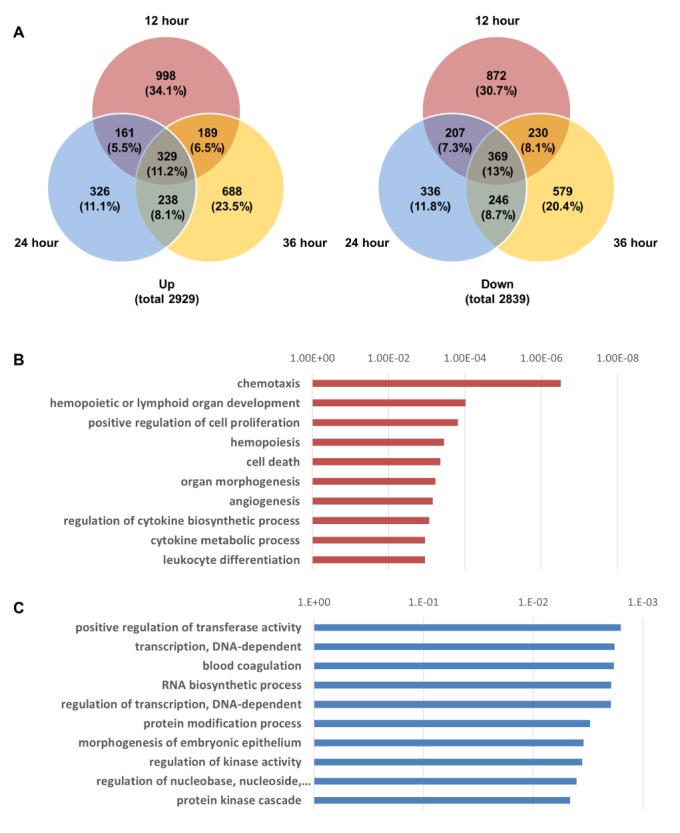
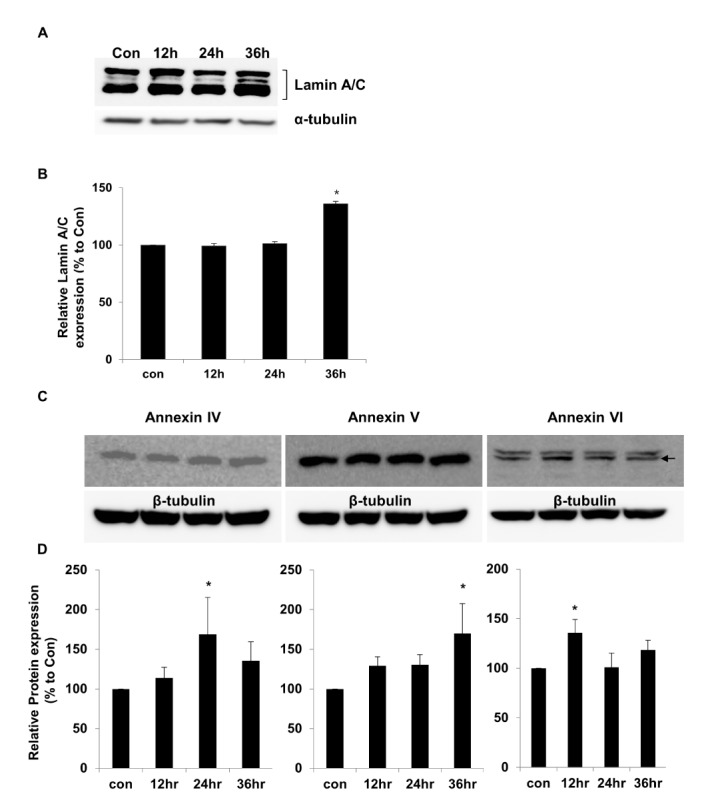
- Activation of Toll-like receptor-4 (TLR-4) in articular chondrocytes increases the catabolic compartment and leads to matrix degradation during the development of osteoarthritis. In this study, we determined the proteomic and genomic alterations in human chondrocytes during lipopolysaccharide (LPS)-induced inflammation to elucidate the underlying mechanisms and consequences of TLR-4 activation. Human chondrocytes were cultured with LPS for 12, 24, and 36 h to induce TLR-4 activation. The TLR-4-induced inflammatory response was confirmed by real-time PCR analysis of increased interleukin-1 beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) expression levels. In TLR-4-activated chondrocytes, proteomic changes were determined by two-dimensional electrophoresis and matrix-assisted laser desorption/ionization-mass spectroscopy analysis, and genomic changes were determined by microarray and gene ontology analyses. Proteomics analysis identified 26 proteins with significantly altered expression levels; these proteins were related to the cytoskeleton and oxidative stress responses. Gene ontology analysis indicated that LPS treatment altered specific functional pathways including "˜chemotaxis', "˜hematopoietic organ development', "˜positive regulation of cell proliferation', and "˜regulation of cytokine biosynthetic process'. Nine of the 26 identified proteins displayed the same increased expression patterns in both proteomics and genomics analyses. Western blot analysis confirmed the LPS-induced increases in expression levels of lamin A/C and annexins 4/5/6. In conclusion, this study identified the time-dependent genomic, proteomic, and functional pathway alterations that occur in chondrocytes during LPS-induced TLR-4 activation. These results provide valuable new insights into the underlying mechanisms that control the development and progression of osteoarthritis.
Keyword
MeSH Terms
-
Annexins*
Blotting, Western
Chondrocytes*
Cytoskeleton
Electrophoresis
Gene Ontology
Genomics
Humans*
Inflammation
Interleukin-1beta
Interleukin-6
Osteoarthritis
Oxidative Stress
Proteomics
Real-Time Polymerase Chain Reaction
Spectrum Analysis
Tumor Necrosis Factor-alpha
Annexins
Interleukin-1beta
Interleukin-6
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009; 1:461–468. PMID: 23015907.
Article2. Campo GM, Avenoso A, Campo S, D'Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie. 2010; 92:204–215. PMID: 19879319.
Article3. Sanchez-Adams J, Leddy HA, McNulty AL, O'Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014; 16:451. PMID: 25182679.
Article4. Gaston JS. Cytokines in arthritis--the ‘big numbers’ move centre stage. Rheumatology (Oxford). 2008; 47:8–12. PMID: 17715172.5. Finckh A, Gabay C. At the horizon of innovative therapy in rheumatology: new biologic agents. Curr Opin Rheumatol. 2008; 20:269–275. PMID: 18388517.
Article6. McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis Rheum. 2007; 56:3033–3042. PMID: 17729298.7. Homandberg GA, Hui F, Wen C. Fibronectin fragment mediated cartilage chondrolysis. I. Suppression by anti-oxidants. Biochim Biophys Acta. 1996; 1317:134–142. PMID: 8950199.
Article8. Hui W, Bell M, Carroll G. Oncostatin M (OSM) stimulates resorption and inhibits synthesis of proteoglycan in porcine articular cartilage explants. Cytokine. 1996; 8:495–500. PMID: 8818547.
Article9. Sandy JD, Neame PJ, Boynton RE, Flannery CR. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991; 266:8683–8685. PMID: 2026585.
Article10. Lai Y, Gallo RL. Toll-like receptors in skin infections and inflammatory diseases. Infect Disord Drug Targets. 2008; 8:144–155. PMID: 18782031.
Article11. O'Neill LA, Dinarello CA. The IL-1 receptor/toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol Today. 2000; 21:206–209. PMID: 10782049.12. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003; 21:335–376. PMID: 12524386.
Article13. Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006; 24:353–389. PMID: 16551253.
Article14. Bobacz K, Sunk IG, Hofstaetter JG, Amoyo L, Toma CD, Akira S, Weichhart T, Saemann M, Smolen JS. Toll-like receptors and chondrocytes: the lipopolysaccharide-induced decrease in cartilage matrix synthesis is dependent on the presence of toll-like receptor 4 and antagonized by bone morphogenetic protein 7. Arthritis Rheum. 2007; 56:1880–1893. PMID: 17530716.
Article15. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006; 124:783–801. PMID: 16497588.
Article16. Hughes AL, Piontkivska H. Functional diversification of the toll-like receptor gene family. Immunogenetics. 2008; 60:249–256. PMID: 18415094.
Article17. Liu-Bryan R, Terkeltaub R. Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous Toll-like receptor 2/Toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein 1 in mice. Arthritis Rheum. 2010; 62:2004–2012. PMID: 20506365.18. Sillat T, Barreto G, Clarijs P, Soininen A, Ainola M, Pajarinen J, Korhonen M, Konttinen YT, Sakalyte R, Hukkanen M, Ylinen P, Nordström DC. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 2013; 84:585–592. PMID: 24237425.
Article19. Kim HA, Cho ML, Choi HY, Yoon CS, Jhun JY, Oh HJ, Kim HY. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006; 54:2152–2163. PMID: 16802353.
Article20. Liu MH, Sun JS, Tsai SW, Sheu SY, Chen MH. Icariin protects murine chondrocytes from lipopolysaccharide-induced inflammatory responses and extracellular matrix degradation. Nutr Res. 2010; 30:57–65. PMID: 20116661.
Article21. Aota Y, An HS, Imai Y, Thonar EJ, Muehleman C, Masuda K. Comparison of cellular response in bovine intervertebral disc cells and articular chondrocytes: effects of lipopolysaccharide on proteoglycan metabolism. Cell Tissue Res. 2006; 326:787–793. PMID: 16788835.
Article22. Haglund L, Bernier SM, Onnerfjord P, Recklies AD. Proteomic analysis of the LPS-induced stress response in rat chondrocytes reveals induction of innate immune response components in articular cartilage. Matrix Biol. 2008; 27:107–118. PMID: 18023983.
Article23. Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharideinduced bone loss associated with inflammation. J Immunol. 2006; 177:1879–1885. PMID: 16849500.24. Matsuguchi T, Takagi K, Musikacharoen T, Yoshikai Y. Gene expressions of lipopolysaccharide receptors, toll-like receptors 2 and 4, are differently regulated in mouse T lymphocytes. Blood. 2000; 95:1378–1385. PMID: 10666214.
Article25. Pålsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004; 113:153–162. PMID: 15379975.
Article26. Otero M, Goldring MB. Cells of the synovium in rheumatoid arthritis. Chondrocytes. Arthritis Res Ther. 2007; 9:220. PMID: 18001488.
Article27. Warda M, Kim HK, Kim N, Youm JB, Kang SH, Park WS, Khoa TM, Kim YH, Han J. Simulated hyperglycemia in rat cardiomyocytes: a proteomics approach for improved analysis of cellular alterations. Proteomics. 2007; 7:2570–2590. PMID: 17647226.
Article28. Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993; 3:327–332. PMID: 15335725.
Article29. Heo HJ, Kim HK, Youm JB, Cho SW, Song IS, Lee SY, Ko TH, Kim N, Ko KS, Rhee BD, Han J. Mitochondrial pyruvate dehydrogenase phosphatase 1 regulates the early differentiation of cardiomyocytes from mouse embryonic stem cells. Exp Mol Med. 2016; 48:e254. PMID: 27538372.
Article30. Won KJ, Lin HY, Jung S, Cho SM, Shin HC, Bae YM, Lee SH, Kim HJ, Jeon BH, Kim B. Antifungal miconazole induces cardiotoxicity via inhibition of APE/Ref-1-related pathway in rat neonatal cardiomyocytes. Toxicol Sci. 2012; 126:298–305. PMID: 22262564.
Article31. Thu VT, Kim HK, Long le T, Thuy TT, Huy NQ, Kim SH, Kim N, Ko KS, Rhee BD, Han J. NecroX-5 exerts anti-inflammatory and anti-fibrotic effects via modulation of the TNFα/Dcn/TGFβ1/Smad2 pathway in hypoxia/reoxygenation-treated rat hearts. Korean J Physiol Pharmacol. 2016; 20:305–314. PMID: 27162485.
Article32. Huang SK, Darfler MM, Nicholl MB, You J, Bemis KG, Tegeler TJ, Wang M, Wery JP, Chong KK, Nguyen L, Scolyer RA, Hoon DS. LC/MS-based quantitative proteomic analysis of paraffin-embedded archival melanomas reveals potential proteomic biomarkers associated with metastasis. PLoS One. 2009; 4:e4430. PMID: 19221597.
Article33. Kim KH. Innate immunity and toll like receptors. Korean J Pediatr. 2004; 47:6–11.34. Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995; 164:383–389. PMID: 8588739.
Article35. Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985; 229:869–871. PMID: 3895437.
Article36. Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990; 171:35–47. PMID: 2104921.
Article37. Huh YH, Lee G, Song WH, Koh JT, Ryu JH. Crosstalk between FLS and chondrocytes is regulated by HIF-2α-mediated cytokines in arthritis. Exp Mol Med. 2015; 47:e197. PMID: 26642431.
Article38. van den Berg WB. Animal models of arthritis. What have we learned? J Rheumatol Suppl. 2005; 72:7–9. PMID: 15660455.39. van den Berg WB, van Riel PL. Uncoupling of inflammation and destruction in rheumatoid arthritis: myth or reality? Arthritis Rheum. 2005; 52:995–999. PMID: 15818666.
Article40. Dasuri K, Antonovici M, Chen K, Wong K, Standing K, Ens W, El-Gabalawy H, Wilkins JA. The synovial proteome: analysis of fibroblast-like synoviocytes. Arthritis Res Ther. 2004; 6:R161–R168. PMID: 15059280.41. Gillette MA, Carr SA. Quantitative analysis of peptides and proteins in biomedicine by targeted mass spectrometry. Nat Methods. 2013; 10:28–34. PMID: 23269374.
Article42. Gu S, Wang T, Chen X. Quantitative proteomic analysis of LPS-induced differential immune response associated with TLR4 Polymorphisms by multiplex amino acid coded mass tagging. Proteomics. 2008; 8:3061–3070. PMID: 18654986.
Article43. Wang H, Ye J. Regulation of energy balance by inflammation: common theme in physiology and pathology. Rev Endocr Metab Disord. 2015; 16:47–54. PMID: 25526866.
Article44. Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY). 2010; 2:361–368. PMID: 20606248.
Article45. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014; 157:1013–1022. PMID: 24855941.
Article46. Rogers KR, Morris CJ, Blake DR. The cytoskeleton and its importance as a mediator of inflammation. Ann Rheum Dis. 1992; 51:565–571. PMID: 1586265.
Article47. Kopecki Z, Ludwig RJ, Cowin AJ. Cytoskeletal regulation of inflammation and its impact on skin blistering disease epidermolysis bullosa acquisita. Int J Mol Sci. 2016; 17:E1116. PMID: 27420054.
Article48. Wickramarachchi DC, Theofilopoulos AN, Kono DH. Immune pathology associated with altered actin cytoskeleton regulation. Autoimmunity. 2010; 43:64–75. PMID: 20001423.
Article49. Mayr M, Chung YL, Mayr U, Yin X, Ly L, Troy H, Fredericks S, Hu Y, Griffiths JR, Xu Q. Proteomic and metabolomic analyses of atherosclerotic vessels from apolipoprotein E-deficient mice reveal alterations in inflammation, oxidative stress, and energy metabolism. Arterioscler Thromb Vasc Biol. 2005; 25:2135–2142. PMID: 16123314.
Article50. Aigner T, Zien A, Hanisch D, Zimmer R. Gene expression in chondrocytes assessed with use of microarrays. J Bone Joint Surg Am. 2003; 85-A(Suppl 2):117–123.
Article51. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998; 95:14863–14868. PMID: 9843981.
Article52. Devauchelle V, Marion S, Cagnard N, Mistou S, Falgarone G, Breban M, Letourneur F, Pitaval A, Alibert O, Lucchesi C, Anract P, Hamadouche M, Ayral X, Dougados M, Gidrol X, Fournier C, Chiocchia G. DNA microarray allows molecular profiling of rheumatoid arthritis and identification of pathophysiological targets. Genes Immun. 2004; 5:597–608. PMID: 15496955.
Article53. Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990; 63:1149–1157. PMID: 2261637.
Article54. Sheikh F, Dickensheets H, Pedras-Vasconcelos J, Ramalingam T, Helming L, Gordon S, Donnelly RP. The interleukin-13 receptor-α1 chain is essential for induction of the alternative macrophage activation pathway by IL-13 but not IL-4. J Innate Immun. 2015; 7:494–505. PMID: 25766112.
Article55. Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008; 102:1307–1318. PMID: 18535268.
Article56. Duque G, Rivas D. Age-related changes in lamin A/C expression in the osteoarticular system: laminopathies as a potential new aging mechanism. Mech Ageing Dev. 2006; 127:378–383. PMID: 16445967.
Article57. Smith ED, Kudlow BA, Frock RL, Kennedy BK. A-type nuclear lamins, progerias and other degenerative disorders. Mech Ageing Dev. 2005; 126:447–460. PMID: 15722103.
Article58. Attur M, Ben-Artzi A, Yang Q, Al-Mussawir HE, Worman HJ, Palmer G, Abramson SB. Perturbation of nuclear lamin A causes cell death in chondrocytes. Arthritis Rheum. 2012; 64:1940–1949. PMID: 22231515.
Article59. Hishiya A, Watanabe K. Progeroid syndrome as a model for impaired bone formation in senile osteoporosis. J Bone Miner Metab. 2004; 22:399–403. PMID: 15316860.
Article60. Helfand BT, Chang L, Goldman RD. Intermediate filaments are dynamic and motile elements of cellular architecture. J Cell Sci. 2004; 117:133–141. PMID: 14676269.
Article61. Ruiz-Romero C, Carreira V, Rego I, Remeseiro S, López-Armada MJ, Blanco FJ. Proteomic analysis of human osteoarthritic chondrocytes reveals protein changes in stress and glycolysis. Proteomics. 2008; 8:495–507. PMID: 18186017.
Article62. Miranda M, Chacón MR, Vidal F, Megia A, Richart C, Veloso S, Saumoy M, Olona C, Vendrell J. LMNA messenger RNA expression in highly active antiretroviral therapy-treated HIV-positive patients. J Acquir Immune Defic Syndr. 2007; 46:384–389. PMID: 18077842.
Article63. Tran JR, Chen H, Zheng X, Zheng Y. Lamin in inflammation and aging. Curr Opin Cell Biol. 2016; 40:124–130. PMID: 27023494.
Article64. Orth K, Chinnaiyan AM, Garg M, Froelich CJ, Dixit VM. The CED-3/ICE-like protease Mch2 is activated during apoptosis and cleaves the death substrate lamin A. J Biol Chem. 1996; 271:16443–16446. PMID: 8663580.
Article65. Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996; 87:171. PMID: 8861900.
Article66. Kaufmann SH, Desnoyers S, Ottaviano Y, Davidson NE, Poirier GG. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993; 53:3976–3985. PMID: 8358726.67. Zhuang J, Dinsdale D, Cohen GM. Apoptosis, in human monocytic THP.1 cells, results in the release of cytochrome c from mitochondria prior to their ultracondensation, formation of outer membrane discontinuities and reduction in inner membrane potential. Cell Death Differ. 1998; 5:953–962. PMID: 9846182.
Article68. Okinaga T, Kasai H, Tsujisawa T, Nishihara T. Role of caspases in cleavage of lamin A/C and PARP during apoptosis in macrophages infected with a periodontopathic bacterium. J Med Microbiol. 2007; 56:1399–1404. PMID: 17893180.
Article69. Osorio FG, Bárcena C, Soria-Valles C, Ramsay AJ, de Carlos F, Cobo J, Fueyo A, Freije JM, López-Otín C. Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012; 26:2311–2324. PMID: 23019125.
Article70. McKenna T, Rosengardten Y, Viceconte N, Baek JH, Grochová D, Eriksson M. Embryonic expression of the common progeroid lamin A splice mutation arrests postnatal skin development. Aging Cell. 2014; 13:292–302. PMID: 24305605.
Article71. Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005; 6:449–461. PMID: 15928709.72. Hayes MJ, Moss SE. Annexins and disease. Biochem Biophys Res Commun. 2004; 322:1166–1170. PMID: 15336964.
Article73. Fatimathas L, Moss SE. Annexins as disease modifiers. Histol Histopathol. 2010; 25:527–532. PMID: 20183805.74. Belluoccio D, Grskovic I, Niehoff A, Schlötzer-Schrehardt U, Rosenbaum S, Etich J, Frie C, Pausch F, Moss SE, Pöschl E, Bateman JF, Brachvogel B. Deficiency of annexins A5 and A6 induces complex changes in the transcriptome of growth plate cartilage but does not inhibit the induction of mineralization. J Bone Miner Res. 2010; 25:141–153. PMID: 19580468.
Article75. Kim SW, Rhee HJ, Ko J, Kim YJ, Kim HG, Yang JM, Choi EC, Na DS. Inhibition of cytosolic phospholipase A2 by annexin I. Specific interaction model and mapping of the interaction site. J Biol Chem. 2001; 276:15712–15719. PMID: 11278580.76. Jia Y, Morand EF, Song W, Cheng Q, Stewart A, Yang YH. Regulation of lung fibroblast activation by annexin A1. J Cell Physiol. 2013; 228:476–484. PMID: 22777765.
Article77. Zanotti G, Malpeli G, Gliubich F, Folli C, Stoppini M, Olivi L, Savoia A, Berni R. Structure of the trigonal crystal form of bovine annexin IV. Biochem J. 1998; 329:101–106. PMID: 9405281.
Article78. Hauptmann R, Maurer-Fogy I, Krystek E, Bodo G, Andree H, Reutelingsperger CP. Vascular anticoagulant beta: a novel human Ca2+/phospholipid binding protein that inhibits coagulation and phospholipase A2 activity. Its molecular cloning, expression and comparison with VAC-alpha. Eur J Biochem. 1989; 185:63–71. PMID: 2530088.79. Mira JP, Dubois T, Oudinet JP, Lukowski S, Russo-Marie F, Geny B. Inhibition of cytosolic phospholipase A2 by annexin V in differentiated permeabilized HL-60 cells Evidence of crucial importance of domain I type II Ca2+-binding site in the mechanism of inhibition. J Biol Chem. 1997; 272:10474–10482. PMID: 9099690.80. Reutelingsperger CP, van Heerde WL. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell Mol Life Sci. 1997; 53:527–532. PMID: 9230931.
Article81. Tokita N, Hasegawa S, Maruyama K, Izumi T, Blankenberg FG, Tait JF, Strauss HW, Nishimura T. 99mTc-Hynic-annexin V imaging to evaluate inflammation and apoptosis in rats with autoimmune myocarditis. Eur J Nucl Med Mol Imaging. 2003; 30:232–238. PMID: 12552341.
Article82. Ewing MM, de Vries MR, Nordzell M, Pettersson K, de Boer HC, van Zonneveld AJ, Frostegård J, Jukema JW, Quax PH. Annexin A5 therapy attenuates vascular inflammation and remodeling and improves endothelial function in mice. Arterioscler Thromb Vasc Biol. 2011; 31:95–101. PMID: 20947818.
Article83. Park JH, Jang JH, Choi EJ, Kim YS, Lee EJ, Jung ID, Han HD, Wu TC, Hung CF, Kang TH, Park YM. Annexin A5 increases survival in murine sepsis model by inhibiting HMGB1-mediated proinflammation and coagulation. Mol Med. 2016; 22.
Article84. Buckley S, Shi W, Xu W, Frey MR, Moats R, Pardo A, Selman M, Warburton D. Increased alveolar soluble annexin V promotes lung inflammation and fibrosis. Eur Respir J. 2015; 46:1417–1429. PMID: 26160872.
Article85. Ravassa S, González A, López B, Beaumont J, Querejeta R, Larman M, Díez J. Upregulation of myocardial Annexin A5 in hypertensive heart disease: association with systolic dysfunction. Eur Heart J. 2007; 28:2785–2791. PMID: 17766279.
Article86. Mollenhauer J, Mok MT, King KB, Gupta M, Chubinskaya S, Koepp H, Cole AA. Expression of anchorin CII (cartilage annexin V) in human young, normal adult, and osteoarthritic cartilage. J Histochem Cytochem. 1999; 47:209–220. PMID: 9889256.
Article87. Rodríguez-García MI, Fernández JA, Rodríguez A, Fernández MP, Gutierrez C, Torre-Alonso JC. Annexin V autoantibodies in rheumatoid arthritis. Ann Rheum Dis. 1996; 55:895–900. PMID: 9014583.88. Kim HJ, Kirsch T. Collagen/annexin V interactions regulate chondrocyte mineralization. J Biol Chem. 2008; 283:10310–10317. PMID: 18281278.
Article89. Ea HK, Monceau V, Camors E, Cohen-Solal M, Charlemagne D, Lioté F. Annexin 5 overexpression increased articular chondrocyte apoptosis induced by basic calcium phosphate crystals. Ann Rheum Dis. 2008; 67:1617–1625. PMID: 18218665.
Article90. Kurtis MS, Tu BP, Gaya OA, Mollenhauer J, Knudson W, Loeser RF, Knudson CB, Sah RL. Mechanisms of chondrocyte adhesion to cartilage: role of beta1-integrins, CD44, and annexin V. J Orthop Res. 2001; 19:1122–1130. PMID: 11781014.91. Turnay J, Olmo N, Lizarbe MA, von der Mark K. Changes in the expression of annexin A5 gene during in vitro chondrocyte differentiation: influence of cell attachment. J Cell Biochem. 2001; 84:132–142. PMID: 11746522.
Article92. Pfander D, Swoboda B, Kirsch T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. Am J Pathol. 2001; 159:1777–1783. PMID: 11696438.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Toll-like Receptor 2 in Cultured Human Keratinocytes: The Effect of Bacterial Antigens, Cytokines and Calcium Concentration
- Nucleic Acid Recognition and Signaling by Toll-like Receptor 9: Compartment-dependent Regulation
- Clinical Significance of Toll-Like Receptor and Toll-Like Receptor Blocker
- Asian Sand Dust Up-Regulates MUC4 Expression in Human Upper Airway Epithelial Cells
- Delphinidin Inhibits LPS-Induced MUC8 and MUC5B Expression Through Toll-like Receptor 4-Mediated ERK1/2 and p38 MAPK in Human Airway Epithelial Cells