Nat Prod Sci.
2017 Mar;23(1):35-39. 10.20307/nps.2017.23.1.35.
Inhibitory Effect of D-chiro-inositol on Both Growth and Recurrence of Breast Tumor from MDA-MB-231 Cancer Cells
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Chungdae-ro 1, Cheongju-si Seowon-gu, Chungcheongbuk-do, Republic of Korea. songs@chungbuk.ac.kr
- KMID: 2376497
- DOI: http://doi.org/10.20307/nps.2017.23.1.35
Abstract
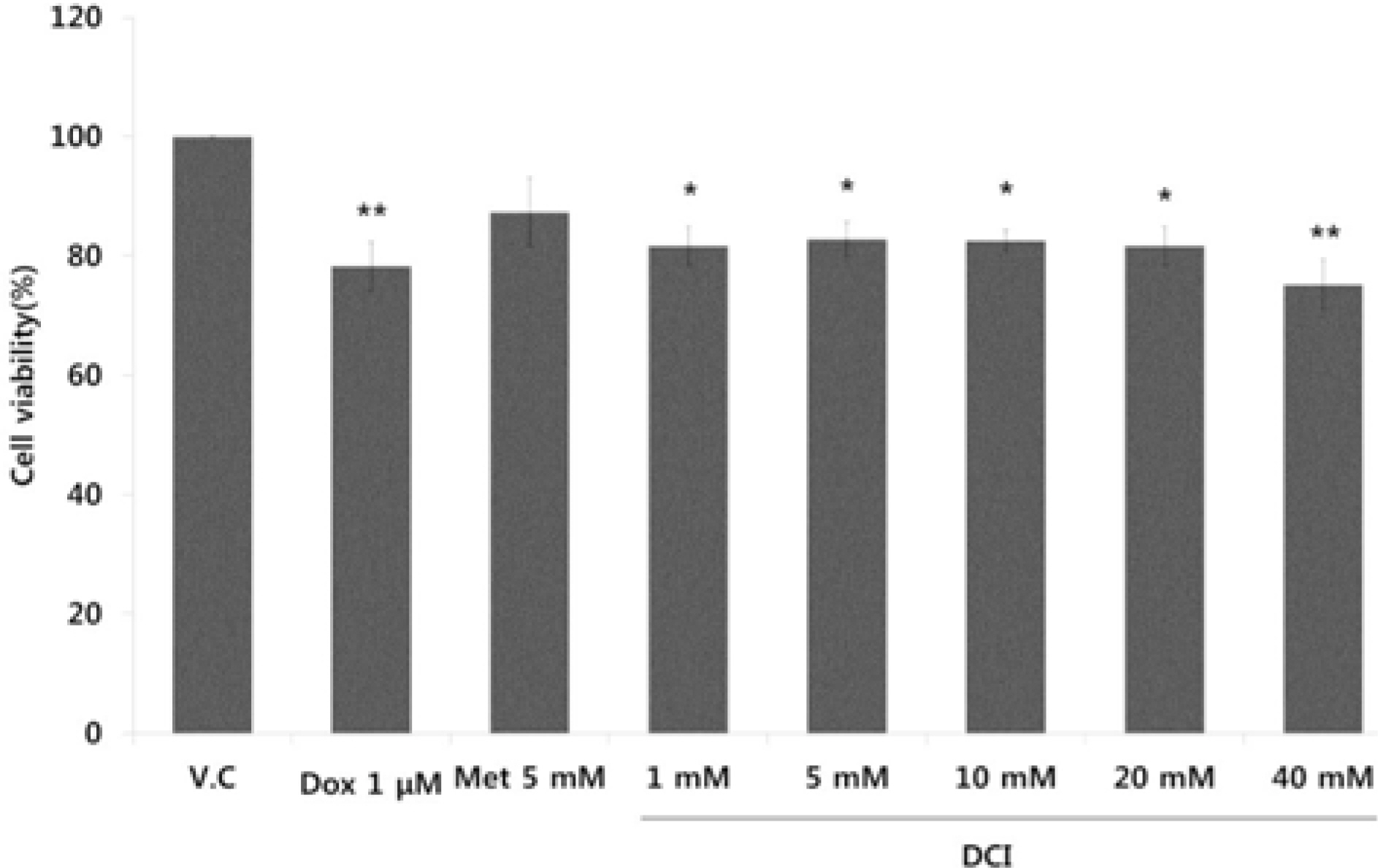
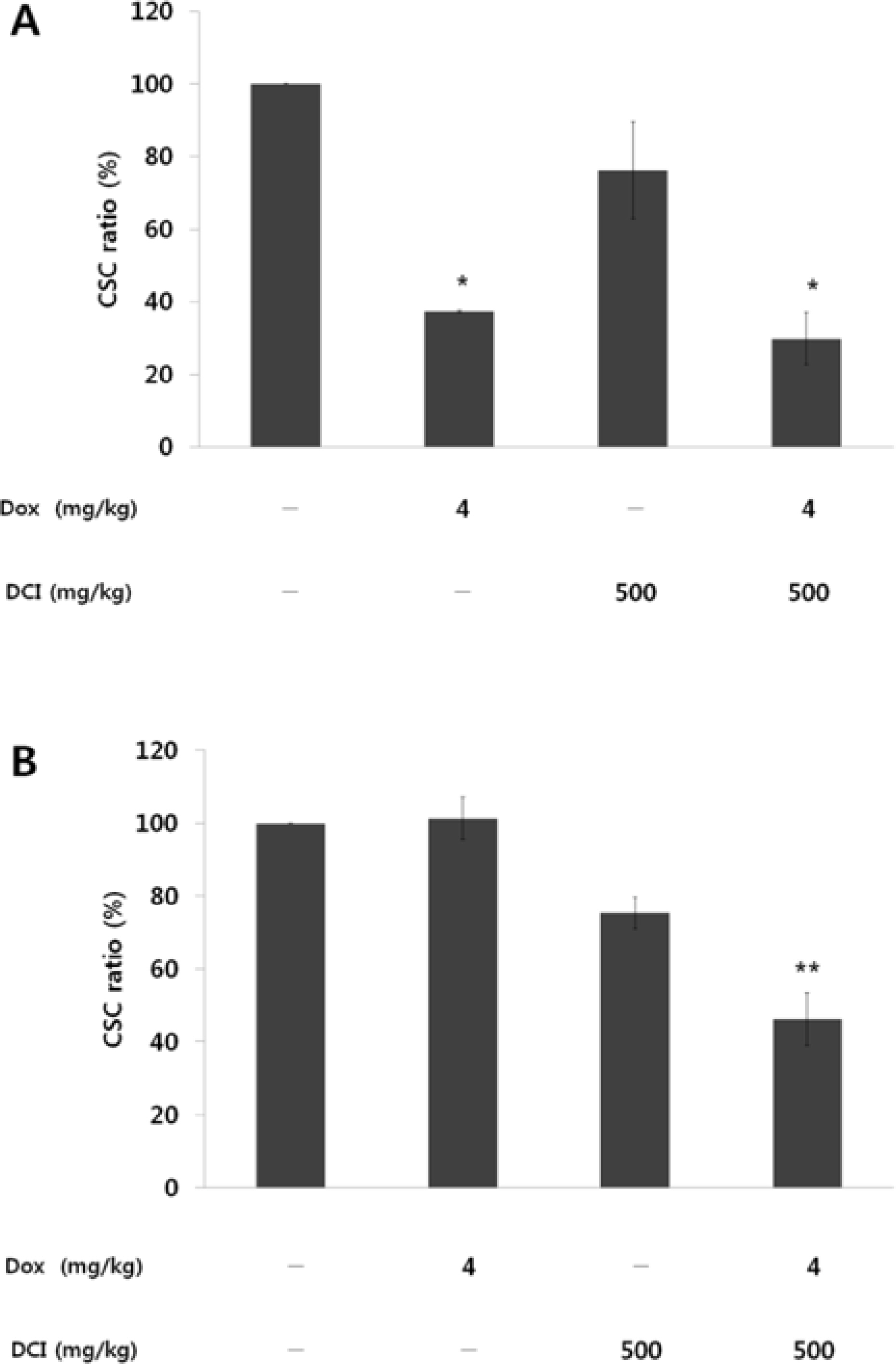
- D-chiro-inositol (DCI) is a secondary messenger in insulin signal transduction. It is produced in vivo from myo-inositol via action of epimerase. In this study, we evaluated antitumor activity of DCI against human breast cancer both in vitro and in vivo. In order to determine the inhibitory effects of DCI on growth of human breast cancer cells (MDA-MB-231), two different assessment methods were implemented: MTT assay and mouse xenograft assay. MTT assay demonstrated downturn in cell proliferation by DCI treatment (1, 5, 10, 20 and 40 mM) groups by 18.3% (p<0.05), 17.2% (p<0.05), 17.5% (p<0.05), 18.4% (p<0.05), and 24.9% (p<0.01), respectively. Also, inhibition of tumor growth was investigated in mouse xenograft model. DCI was administered orally at the dose of 500 mg/kg and 1000 mg/kg body weight to treat nude mouse for 45 consecutive days. On the 45th day, tumor growth of DCI (500 mg/kg and 1000 mg/kg) groups was suppressed by 22.1% and 67.6% as mean tumor volumes were 9313.8 ± 474.1 mm³ and 3879.1 ± 1044.1 mm³, respectively. Furthermore, breast cancer stem cell (fCSC) phenotype (CD44âº/CD24â») was measured using flow cytometry. On the 46th day, CSC ratios of DCI (500 mg/kg) and co-treatment with doxorubicin (4 mg/kg) and DCI (500 mg/kg) group decreased by 24.7% and 53.9% (p<0.01), respectively. Finally, from tumor recurrence assay, delay of 5 days in the co-treatment group compared to doxorubicin (4 mg/kg) alone group was observed. Based on these findings, we propose that DCI holds potential as an anti-cancer drug for treatment of breast cancer.
MeSH Terms
Figure
Reference
-
References
(1). Larner J.Int. J. Exp. Diabetes Res. 2002. 3:47–60.(2). Nestler J. E.., Jakubowicz D. J.., Reamer P.., Gunn R. D.., Allan G. N.Engl. J. Med. 1999. 340:1314–1320.(3). Jung K. W.., Won Y. J.., Oh C. M.., Kong H. J.., Cho H.., Lee D. H.., Lee K. H.Cancer Res. Treat. 2015. 47:142–148.(4). Jung K. W.., Won Y. J.., Kong H. J.., Oh C. M.., Cho H.., Lee D. H.., Lee K. H.Cancer Res. Treat. 2015. 47:127–141.(5). Moody S. E.., Perez D.., Pan T. C.., Sarkisian C. J.., Portocarrero C. P.., Sterner C. J.., Notorfrancesco K. L.., Cardiff R. D.., Chodosh L. A.Cancer Cell. 2005. 8:197–209.(6). Lacerda L.., Pusztai L.., Woodward W. A.Drug Resist. Updat. 2010. 13:99–108.(7). McDermott S. P.., Wicha M. S.Mol. Oncol. 2010. 4:404–419.(8). Dave B.., Mittal V.., Tan N. M.., Chang J. C.Breast Cancer Res. 2012. 14:202.(9). Barkan D.., Chambers A. F.Clin. Cancer Res. 2011. 17:7219–7223.(10). Reedijk M.., Pinnaduwage D.., Dickson B. C.., Mulligan A. M.., Zhang H.., Bull S. B.., O'Malley F. P.., Egan S. E.., Andrulis I. L.Breast Cancer Res. Treat. 2008. 111:439–448.(11). Gangopadhyay S.., Nandy A.., Hor P.., Mukhopadhyay A.Clin. Breast Cancer. 2013. 13:7–15.(12). Karamboulas C.., Ailles L.Biochim. Biophys. Acta. 2013. 1830:2481–2495.(13). Izrailit J.., Reedijk M.Cancer Lett. 2012. 317:115–126.(14). Hassounah N. B.., Bunch T. A.., McDermott K. M.Clin. Cancer Res. 2012. 18:2429–2435.(15). Tang J.., Ahmad A.., Sarkar F. H.Int. J. Mol. Sci. 2012. 13:13414–13437.(16). Li L.., Xie X.., Luo J.., Liu M.., Xi S.., Guo J.., Kong Y.., Wu M.., Gao J.., Xie Z.., Tang J.., Wang X.., Wei W.., Yang M.., Hung MC.., Xie X.Mol. Ther. 2012. 20:2326–2334.(17). Sanguinetti A.., Bistoni G.., Avenia N. G.Chir. 2011. 32:438–446.(18). Velasco-Velázquez M. A.., Homsi N.., De La Fuente M.., Pestell R. G.Int. J. Biochem. Cell Biol. 2012. 44:573–577.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Curcumin on Cancer Invasion and Matrix Metalloproteinase-9 Activity in MDA-MB-231 Human Breast Cancer Cell
- Action and Signaling of Lysophosphatidylethanolamine in MDA-MB-231 Breast Cancer Cells
- Comparative Studies on the Polyarnine Involvement in MCF - 7 and MDA - MB - 231 Breast Cancer Cell Proliferation
- The anti-cancer effect of pomegranate-derived nanovesicles on MDA-MB-231 breast cancer cells
- Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells