Korean J Physiol Pharmacol.
2017 Jan;21(1):55-64. 10.4196/kjpp.2017.21.1.55.
Dehydroevodiamine·HCl enhances cognitive function in memory-impaired rat models
- Affiliations
-
- 1Department of Microbiology, College of Natural Science, Dankook University, Cheonan 31116, Korea.
- 2Department of Nursing, College of Nursing, Gachon University, Incheon 21936, Korea. kykim@gachon.ac.kr
- 3Department of Pharmacology, College of Medicine, Neuroscience Research Institute (NRI), Gachon University, Incheon 21565, Korea. yhsuh@gachon.ac.kr
- KMID: 2371068
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.55
Abstract
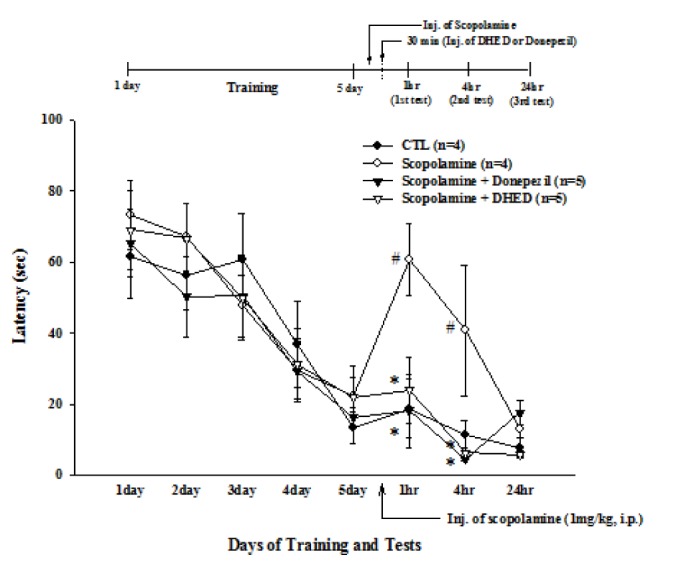
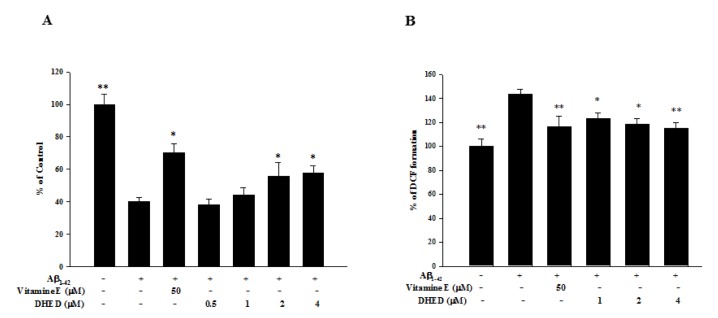
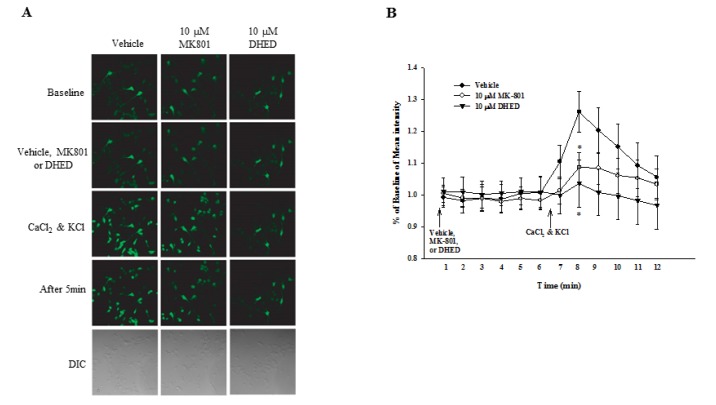
- Progressive memory impairment such as that associated with depression, stroke, and Alzheimer's disease (AD) can interfere with daily life. In particular, AD, which is a progressive neurodegenerative disorder, prominently features a memory and learning impairment that is related to changes in acetylcholine and abnormal β-amyloid (Aβ) deposition in the brain. In the present study, we investigated the effects of dehydroevodiamine·HCl (DHED) on cognitive improvement and the related mechanism in memory-impaired rat models, namely, a scopolamine-induced amnesia model and a Aβâ‚â‚‹â‚„â‚‚-infused model. The cognitive effects of DHED were measured using a water maze test and a passive avoidance test in the memory-impaired rat models. The results demonstrate that DHED (10 mg/kg, p.o.) and Donepezil (1 mg/kg, p.o.) ameliorated the spatial memory impairment in the scopolamine-induced amnestic rats. Moreover, DHED significantly improved learning and memory in the Aβâ‚â‚‹â‚„â‚‚-infused rat model. Furthermore, the mechanism of these behavioral effects of DHED was investigated using a cell viability assay, reactive oxygen species (ROS) measurement, and intracellular calcium measurement in primary cortical neurons. DHED reduced neurotoxicity and the production of Aβ-induced ROS in primary cortical neurons. In addition, similar to the effect of MK801, DHED decreased intracellular calcium levels in primary cortical neurons. Our results suggest that DHED has strong protective effects against cognitive impairments through its antioxidant activity and inhibition of neurotoxicity and intracellular calcium. Thus, DHED may be an important therapeutic agent for memory-impaired symptoms.
MeSH Terms
-
Acetylcholine
Alzheimer Disease
Amnesia
Animals
Brain
Calcium
Cell Survival
Cognition Disorders
Cognition*
Depression
Dizocilpine Maleate
Learning
Memory
Models, Animal*
Neurodegenerative Diseases
Neurons
Rats*
Reactive Oxygen Species
Scopolamine Hydrobromide
Spatial Memory
Stroke
Water
Acetylcholine
Calcium
Dizocilpine Maleate
Reactive Oxygen Species
Scopolamine Hydrobromide
Water
Figure
Reference
-
1. Roussel M, Martinaud O, Henon H, Vercelletto M, Bindschadler C, Joseph PA, Robert P, Labauge P, Godefroy O, Group GS. The behavioral and cognitive executive disorders of stroke: the GREFEX study. PLoS One. 2016; 11:e0147602. PMID: 26824746.
Article2. Post RM. Epigenetic basis of sensitization to stress, affective episodes, and stimulants: implications for illness progression and prevention. Bipolar Disord. 2016; 18:315–324. PMID: 27346321.
Article3. Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer's disease. Pharmacol Rev. 2002; 54:469–525. PMID: 12223532.4. Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999; 66:137–147. PMID: 10071091.
Article5. Holston EC. The electrophysiological phenomenon of alzheimer's disease: a psychopathology theory. Issues Ment Health Nurs. 2015; 36:603–613. PMID: 26379134.
Article6. Guntupalli S, Widagdo J, Anggono V. Amyloid-β-induced dysregulation of AMPA receptor trafficking. Neural Plast. 2016; 2016:3204519. PMID: 27073700.
Article7. Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer's disease. Biomed Rep. 2016; 4:519–522. PMID: 27123241.
Article8. Magi S, Castaldo P, Macri ML, Maiolino M, Matteucci A, Bastioli G, Gratteri S, Amoroso S, Lariccia V. Intracellular calcium dysregulation: implications for Alzheimer's disease. Biomed Res Int. 2016; 2016:6701324. PMID: 27340665.
Article9. Haider A, Inam W, Khan SA, Hifza , Mahmood W, Abbas G. β-glucan attenuated scopolamine induced cognitive impairment via hippocampal acetylcholinesterase inhibition in rats. Brain Res. 2016; 1644:141–148. PMID: 27180103.
Article10. Pistollato F, Ohayon EL, Lam A, Langley GR, Novak TJ, Pamies D, Perry G, Trushina E, Williams RS, Roher AE, Hartung T, Harnad S, Barnard N, Morris MC, Lai MC, Merkley R, Chandrasekera PC. Alzheimer disease research in the 21st century: past and current failures, new perspectives and funding priorities. Oncotarget. 2016; 7:38999–39016. PMID: 27229915.
Article11. Soodi M, Naghdi N, Hajimehdipoor H, Choopani S, Sahraei E. Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated rats. Res Pharm Sci. 2014; 9:107–114. PMID: 25657779.12. Hashimoto M, Tozawa R, Katakura M, Shahdat H, Haque AM, Tanabe Y, Gamoh S, Shido O. Protective effects of prescription n-3 fatty acids against impairment of spatial cognitive learning ability in amyloid β-infused rats. Food Funct. 2011; 2:386–394. PMID: 21894325.
Article13. Yun HM, Kim HS, Park KR, Shin JM, Kang AR, il Lee K, Song S, Kim YB, Han SB, Chung HM, Hong JT. Placenta-derived mesenchymal stem cells improve memory dysfunction in an Aβ1-42-infused mouse model of Alzheimer's disease. Cell Death Dis. 2013; 4:e958. PMID: 24336078.
Article14. Choi Y, Kim HS, Shin KY, Kim EM, Kim M, Kim HS, Park CH, Jeong YH, Yoo J, Lee JP, Chang KA, Kim S, Suh YH. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer's disease models. Neuropsychopharmacology. 2007; 32:2393–2404. PMID: 17406652.
Article15. Joo Y, Kim HS, Woo RS, Park CH, Shin KY, Lee JP, Chang KA, Kim S, Suh YH. Mefenamic acid shows neuroprotective effects and improves cognitive impairment in in vitro and in vivo Alzheimer's disease models. Mol Pharmacol. 2006; 69:76–84. PMID: 16223958.
Article16. Decker M. Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine. Eur J Med Chem. 2005; 40:305–313. PMID: 15725500.
Article17. Unsworth WP, Kitsiou C, Taylor RJ. An expedient protecting-group-free total synthesis of (±)-dievodiamine. Org Lett. 2013; 15:3302–3305. PMID: 23786450.
Article18. Park CH, Lee YJ, Lee SH, Choi SH, Kim HS, Jeong SJ, Kim SS, Suh YH. Dehydroevodiamine.HCl prevents impairment of learning and memory and neuronal loss in rat models of cognitive disturbance. J Neurochem. 2000; 74:244–253. PMID: 10617126.
Article19. Fang J, Liu R, Tian Q, Hong XP, Wang SH, Cao FY, Pan XP, Wang JZ. Dehydroevodiamine attenuates calyculin A-induced tau hyperphosphorylation in rat brain slices. Acta Pharmacol Sin. 2007; 28:1717–1723. PMID: 17959021.
Article20. Peng JH, Zhang CE, Wei W, Hong XP, Pan XP, Wang JZ. Dehydroevodiamine attenuates tau hyperphosphorylation and spatial memory deficit induced by activation of glycogen synthase kinase-3 in rats. Neuropharmacology. 2007; 52:1521–1527. PMID: 17434540.
Article21. Yang MC, Wu SL, Kuo JS, Chen CF. The hypotensive and negative chronotropic effects of dehydroevodiamine. Eur J Pharmacol. 1990; 182:537–542. PMID: 2226622.
Article22. van der Staay FJ, Bouger PC. Effects of the cholinesterase inhibitors donepezil and metrifonate on scopolamine-induced impairments in the spatial cone field orientation task in rats. Behav Brain Res. 2005; 156:1–10. PMID: 15474645.
Article23. Nitta A, Fukuta T, Hasegawa T, Nabeshima T. Continuous infusion of beta-amyloid protein into the rat cerebral ventricle induces learning impairment and neuronal and morphological degeneration. Jpn J Pharmacol. 1997; 73:51–57. PMID: 9032134.24. Itoh A, Nitta A, Nadai M, Nishimura K, Hirose M, Hasegawa T, Nabeshima T. Dysfunction of cholinergic and dopaminergic neuronal systems in beta-amyloid protein--infused rats. J Neurochem. 1996; 66:1113–1117. PMID: 8769873.25. Oka J, Suzuki E, Kondo Y. Endogenous GLP-1 is involved in beta-amyloid protein-induced memory impairment and hippocampal neuronal death in rats. Brain Res. 2000; 878:194–198. PMID: 10996151.26. Shin KY, Lee GH, Park CH, Kim HJ, Park SH, Kim S, Kim HS, Lee KS, Won BY, Lee HG, Choi JH, Suh YH. A novel compound, maltolyl p-coumarate, attenuates cognitive deficits and shows neuroprotective effects in vitro and in vivo dementia models. J Neurosci Res. 2007; 85:2500–2511. PMID: 17600377.27. Yamada K, Tanaka T, Han D, Senzaki K, Kameyama T, Nabeshima T. Protective effects of idebenone and alpha-tocopherol on beta-amyloid-(1-42)-induced learning and memory deficits in rats: implication of oxidative stress in beta-amyloid-induced neurotoxicity in vivo. Eur J Neurosci. 1999; 11:83–90. PMID: 9987013.28. Shen Z, Wang G, Lin SZ. Two-way shuttlebox avoidance conditioning and brain NADH in rats. Physiol Behav. 1990; 48:515–517. PMID: 2075201.
Article29. Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999; 27:612–616. PMID: 10490282.30. Nahm WK, Philpot BD, Adams MM, Badiavas EV, Zhou LH, Butmarc J, Bear MF, Falanga V. Significance of N-methyl-D-aspartate (NMDA) receptor-mediated signaling in human keratinocytes. J Cell Physiol. 2004; 200:309–317. PMID: 15174101.31. Roychowdhury S, Noack J, Engelmann M, Wolf G, Horn TF. AMPA receptor-induced intracellular calcium response in the paraventricular nucleus is modulated by nitric oxide: calcium imaging in a hypothalamic organotypic cell culture model. Nitric Oxide. 2006; 14:290–299. PMID: 16442320.
Article32. Kim HJ, Shin KY, Chang KA, Ahn S, Choi HS, Kim HS, Suh YH. Dehydroevodiamine·HCl improves stress-induced memory impairments and depression like behavior in rats. Korean J Physiol Pharmacol. 2014; 18:55–59. PMID: 24634597.
Article33. Craft JM, Van Eldik LJ, Zasadzki M, Hu W, Watterson DM. Aminopyridazines attenuate hippocampus-dependent behavioral deficits induced by human beta-amyloid in a murine model of neuroinflammation. J Mol Neurosci. 2004; 24:115–122. PMID: 15314259.34. Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982; 217:408–414. PMID: 7046051.
Article35. Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000; 163:495–529. PMID: 10833325.
Article36. Lindner MD, Hogan JB, Hodges DB Jr, Orie AF, Chen P, Corsa JA, Leet JE, Gillman KW, Rose GM, Jones KM, Gribkoff VK. Donepezil primarily attenuates scopolamine-induced deficits in psychomotor function, with moderate effects on simple conditioning and attention, and small effects on working memory and spatial mapping. Psychopharmacology (Berl). 2006; 188:629–640. PMID: 17004085.
Article37. Unzeta M, Esteban G, Bolea I, Fogel WA, Ramsay RR, Youdim MB, Tipton KF, Marco-Contelles J. Multi-target directed Donepezil-like ligands for Alzheimer's disease. Front Neurosci. 2016; 10:205. PMID: 27252617.
Article38. Kotani S, Yamauchi T, Teramoto T, Ogura H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact. 2008; 175:227–230. PMID: 18501884.
Article39. Nakamura S, Murayama N, Noshita T, Annoura H, Ohno T. Progressive brain dysfunction following intracerebroventricular infusion of beta(1-42)-amyloid peptide. Brain Res. 2001; 912:128–136. PMID: 11532428.
Article40. Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D'Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B. Neurotoxicity of Alzheimer's disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010; 29:3408–3420. PMID: 20818335.
Article41. Subbarao KV, Richardson JS, Ang LC. Autopsy samples of Alzheimer's cortex show increased peroxidation in vitro. J Neurochem. 1990; 55:342–345. PMID: 2355227.
Article42. Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, Bozner P. Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer's disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol. 1998; 152:871–877. PMID: 9546346.43. Misonou H, Morishima-Kawashima M, Ihara Y. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry. 2000; 39:6951–6959. PMID: 10841777.44. Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992; 186:944–950. PMID: 1497677.45. LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer's disease. Nat Rev Neurosci. 2002; 3:862–872. PMID: 12415294.
Article46. Liang J, Kulasiri D, Samarasinghe S. Ca2+ dysregulation in the endoplasmic reticulum related to Alzheimer's disease: a review on experimental progress and computational modeling. Biosystems. 2015; 134:1–15. PMID: 25998697.47. Birnbaum JH, Bali J, Rajendran L, Nitsch RM, Tackenberg C. Calcium flux-independent NMDA receptor activity is required for Aβ oligomer-induced synaptic loss. Cell Death Dis. 2015; 6:e1791. PMID: 26086964.
Article48. Hermes M, Eichhoff G, Garaschuk O. Intracellular calcium signalling in Alzheimer's disease. J Cell Mol Med. 2010; 14:30–41. PMID: 19929945.
Article49. Ferreira IL, Bajouco LM, Mota SI, Auberson YP, Oliveira CR, Rego AC. Amyloid beta peptide 1-42 disturbs intracellular calcium homeostasis through activation of GluN2B-containing N-methyl-d-aspartate receptors in cortical cultures. Cell Calcium. 2012; 51:95–106. PMID: 22177709.
Article