Ann Dermatol.
2017 Feb;29(1):6-12. 10.5021/ad.2017.29.1.6.
Kinetin Improves Barrier Function of the Skin by Modulating Keratinocyte Differentiation Markers
- Affiliations
-
- 1Korea Institute for Skin and Clinical Sciences, Konkuk University, Seoul, Korea. gongju72@hanmail.net
- 2KISCS Incorporated, Cheongju, Korea.
- 3Orangewood Christian School, Maitland, FL, USA.
- 4Department of Dermatology, Konkuk University School of Medicine, Seoul, Korea. kjahn@kuh.ac.kr
- KMID: 2368021
- DOI: http://doi.org/10.5021/ad.2017.29.1.6
Abstract
- BACKGROUND
Kinetin is a plant hormone that regulates growth and differentiation. Keratinocytes, the basic building blocks of the epidermis, function in maintaining the skin barrier.
OBJECTIVE
We examined whether kinetin induces skin barrier functions in vitro and in vivo.
METHODS
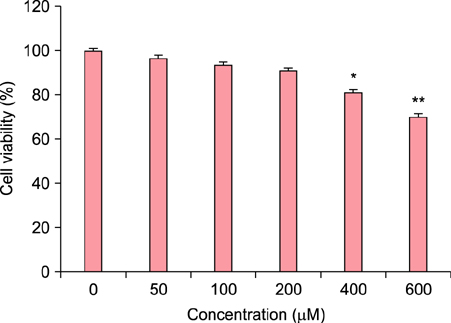
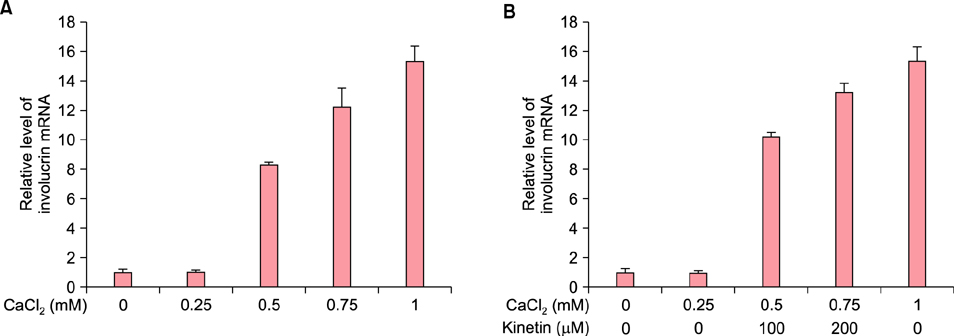
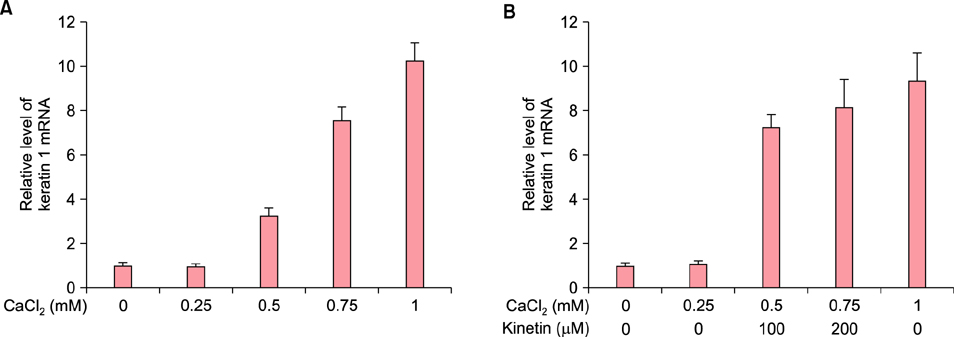
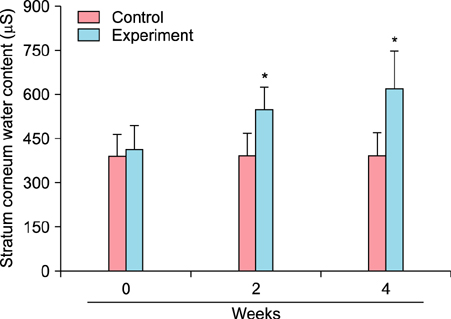
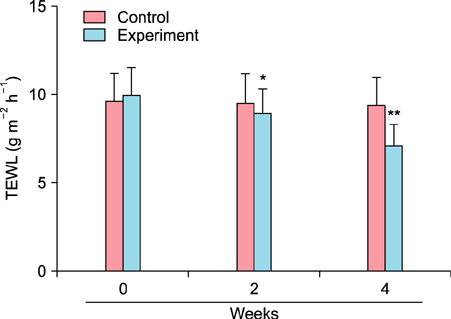
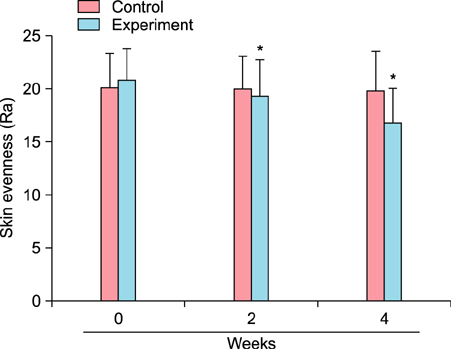
To evaluate the efficacy of kinetin at the cellular level, expression of keratinocyte differentiation markers was assessed. Moreover, we examined the clinical efficacy of kinetin by evaluating skin moisture, transepidermal water loss (TEWL), and skin surface roughness in patients who used kinetin-containing cream. We performed quantitative real-time polymerase chain reaction to measure the expression of keratinocyte differentiation markers in HaCaT cells following treatment. A clinical trial was performed to assess skin moisture, TEWL, and evenness of skin texture in subjects who used kinetin-containing cream for 4 weeks.
RESULTS
Kinetin increased involucrin, and keratin 1 mRNA in HaCaT cells. Moreover, use of a kinetin-containing cream improved skin moisture and TEWL while decreasing roughness of skin texture.
CONCLUSION
Kinetin induced the expression of keratinocyte differentiation markers, suggesting that it may affect differentiation to improve skin moisture content, TEWL, and other signs of skin aging. Therefore, kinetin is a potential new component for use in cosmetics as an anti-aging agent that improves the barrier function of skin.
MeSH Terms
Figure
Reference
-
1. Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002; 123:801–810.
Article2. Zouboulis CC, Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011; 29:3–14.
Article3. Hashizume H. Skin aging and dry skin. J Dermatol. 2004; 31:603–609.
Article4. Farage MA, Miller KW, Elsner P, Maibach HI. Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci. 2008; 30:87–95.
Article5. Elsner P, Fluhr JW, Gehring W, Kerscher MJ, Krutmann J, Lademann J, et al. Anti-aging data and support claims--consensus statement. J Dtsch Dermatol Ges. 2011; 9:Suppl 3. S1–S32.6. Landau M. Exogenous factors in skin aging. Curr Probl Dermatol. 2007; 35:1–13.
Article7. Miller CO, Skoog F, von Saltza MH, Strong FM. Kinetin, a cell division factor from deoxyribonucleic acid. J Am Chem Soc. 1955; 77:1392.8. Amasino R. 1955: kinetin arrives: the 50th anniversary of a new plant hormone. Plant Physiol. 2005; 138:1177–1184.
Article9. Barciszewski J, Siboska GE, Pedersen BO, Clark BF, Rattan SI. Evidence for the presence of kinetin in DNA and cell extracts. FEBS Lett. 1996; 393:197–200.
Article10. Barciszewski J, Mielcarek M, Stobiecki M, Siboska G, Clark BF. Identification of 6-furfuryladenine (kinetin) in human urine. Biochem Biophys Res Commun. 2000; 279:69–73.
Article11. Barciszewski J, Siboska GE, Pedersen BO, Clark BF, Rattan SI. Furfural, a precursor of the cytokinin hormone kinetin, and base propenals are formed by hydroxyl radical damage of DNA. Biochem Biophys Res Commun. 1997; 238:317–319.
Article12. Rattan SI, Clark BF. Kinetin delays the onset of ageing characteristics in human fibroblasts. Biochem Biophys Res Commun. 1994; 201:665–672.13. Sharma SP, Kaur P, Rattan SI. Plant growth hormone kinetin delays ageing, prolongs the lifespan and slows down development of the fruitfly Zaprionus paravittiger. Biochem Biophys Res Commun. 1995; 216:1067–1071.
Article14. Sharma SP, Kaur J, Rattan SI. Increased longevity of kinetin-fed Zaprionus fruitflies is accompanied by their reduced fecundity and enhanced catalase activity. Biochem Mol Biol Int. 1997; 41:869–875.
Article15. Olsen A, Siboska GE, Clark BF, Rattan SI. N(6)-Furfury-ladenine, kinetin, protects against Fenton reaction-mediated oxidative damage to DNA. Biochem Biophys Res Commun. 1999; 265:499–502.
Article16. Verbeke P, Siboska GE, Clark BF, Rattan SI. Kinetin inhibits protein oxidation and glycoxidation in vitro. Biochem Biophys Res Commun. 2000; 276:1265–1270.
Article17. Hsiao G, Shen MY, Lin KH, Chou CY, Tzu NH, Lin CH, et al. Inhibitory activity of kinetin on free radical formation of activated platelets in vitro and on thrombus formation in vivo. Eur J Pharmacol. 2003; 465:281–287.
Article18. Sheu JR, Hsiao G, Shen MY, Chou CY, Lin CH, Chen TF, et al. Inhibitory mechanisms of kinetin, a plant growthpromoting hormone, in platelet aggregation. Platelets. 2003; 14:189–196.
Article19. Lee JH, Chung KY, Bang D, Lee KH. Searching for aging-related proteins in human dermal microvascular endothelial cells treated with anti-aging agents. Proteomics. 2006; 6:1351–1361.
Article20. Ishii Y, Sakai S, Honma Y. Cytokinin-induced differentiation of human myeloid leukemia HL-60 cells is associated with the formation of nucleotides, but not with incorporation into DNA or RNA. Biochim Biophys Acta. 2003; 1643:11–24.
Article21. Berge U, Kristensen P, Rattan SI. Kinetin-induced differentiation of normal human keratinocytes undergoing aging in vitro. Ann N Y Acad Sci. 2006; 1067:332–336.
Article22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article23. Muendnich K, Spann W, Reichenbach M, JACOBI . The clinical and forensic significance of complications after puncture of the maxillary sinuses. HNO. 1959; 8:24–28.24. Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol. 1980; 75:500–507.
Article25. Wikramanayake TC, Stojadinovic O, Tomic-Canic M. Epidermal differentiation in barrier maintenance and wound healing. Adv Wound Care (New Rochelle). 2014; 3:272–280.
Article26. Contet-Audonneau JL, Jeanmaire C, Pauly G. A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol. 1999; 140:1038–1047.
Article27. Chiu PC, Chan CC, Lin HM, Chiu HC. The clinical anti-aging effects of topical kinetin and niacinamide in Asians: a randomized, double-blind, placebo-controlled, split-face comparative trial. J Cosmet Dermatol. 2007; 6:243–249.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Citronellic Acid Improves Skin Barrier Function by Activation of PPAR-α
- Stimulation of keratinocyte differentiation by extract of combined medicinal plant
- Rab25 Deficiency Perturbs Epidermal Differentiation and Skin Barrier Function in Mice
- Enhancement of Keratinocyte Differentiation by Rose Absolute Oil
- Skin Barrier and Calcium