Ann Dermatol.
2018 Jun;30(3):265-275. 10.5021/ad.2018.30.3.265.
Skin Barrier and Calcium
- Affiliations
-
- 1Department of Dermatology and Cutaneous Biology Research Institute, Yonsei University College of Medicine, Seoul, Korea. ydshderm@gmail.com
- KMID: 2419166
- DOI: http://doi.org/10.5021/ad.2018.30.3.265
Abstract
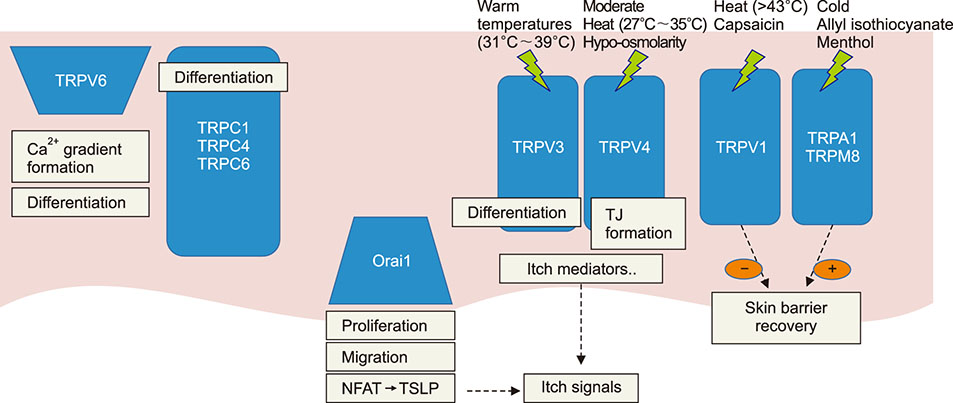
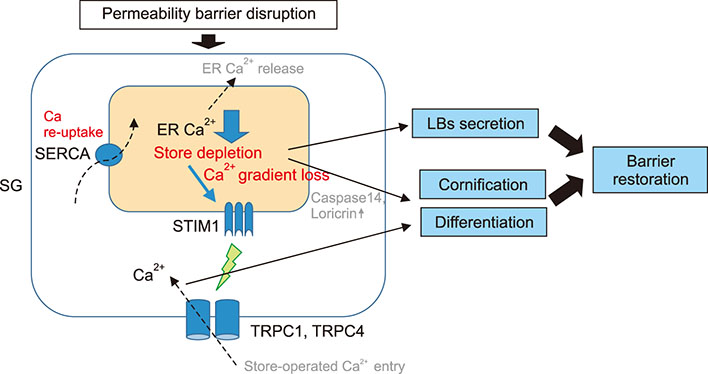
- Epidermal barrier formation and the maintenance of barrier homeostasis are essential to protect us from the external environments and organisms. Moreover, impaired keratinocytes differentiation and dysfunctional skin barrier can be the primary causes or aggravating factors for many inflammatory skin diseases including atopic dermatitis and psoriasis. Therefore, understanding the regulation mechanisms of keratinocytes differentiation and skin barrier homeostasis is important to understand many skin diseases and establish an effective treatment strategy. Calcium ions (Ca²âº) and their concentration gradient in the epidermis are essential in regulating many skin functions, including keratinocyte differentiation, skin barrier formation, and permeability barrier homeostasis. Recent studies have suggested that the intracellular Ca²âº stores such as the endoplasmic reticulum (ER) are the major components that form the epidermal calcium gradient and the ER calcium homeostasis is crucial for regulating keratinocytes differentiation, intercellular junction formation, antimicrobial barrier, and permeability barrier homeostasis. Thus, both Ca²âº release from intracellular stores, such as the ER and Ca²âº influx mechanisms are important in skin barrier. In addition, growing evidences identified the functional existence and the role of many types of calcium channels which mediate calcium flux in keratinocytes. In this review, the origin of epidermal calcium gradient and their role in the formation and regulation of skin barrier are focused. We also focus on the role of ER calcium homeostasis in skin barrier. Furthermore, the distribution and role of epidermal calcium channels, including transient receptor potential channels, store-operated calcium entry channel Orai1, and voltage-gated calcium channels in skin barrier are discussed.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Nootkatol prevents ultraviolet radiation-induced photoaging
via ORAI1 and TRPV1 inhibition in melanocytes and keratinocytes
Joo Han Woo, Da Yeong Nam, Hyun Jong Kim, Phan Thi Lam Hong, Woo Kyung Kim, Joo Hyun Nam
Korean J Physiol Pharmacol. 2021;25(1):87-94. doi: 10.4196/kjpp.2021.25.1.87.
Reference
-
1. Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985; 84:508–512.
Article2. Menon GK, Elias PM. Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch Dermatol. 1991; 127:57–63.
Article3. Elias P, Ahn S, Brown B, Crumrine D, Feingold KR. Origin of the epidermal calcium gradient: regulation by barrier status and role of active vs passive mechanisms. J Invest Dermatol. 2002; 119:1269–1274.
Article4. Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992; 89:530–538.
Article5. Lee SH, Elias PM, Feingold KR, Mauro T. A role for ions in barrier recovery after acute perturbation. J Invest Dermatol. 1994; 102:976–979.
Article6. Menon GK, Elias PM, Lee SH, Feingold KR. Localization of calcium in murine epidermis following disruption and repair of the permeability barrier. Cell Tissue Res. 1992; 270:503–512.
Article7. Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992; 98:279–289.
Article8. Yuspa SH, Kilkenny AE, Steinert PM, Roop DR. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989; 109:1207–1217.
Article9. Behne MJ, Sanchez S, Barry NP, Kirschner N, Meyer W, Mauro TM, et al. Major translocation of calcium upon epidermal barrier insult: imaging and quantification via FLIM/Fourier vector analysis. Arch Dermatol Res. 2011; 303:103–115.
Article10. Celli A, Sanchez S, Behne M, Hazlett T, Gratton E, Mauro T. The epidermal Ca(2+) gradient: measurement using the phasor representation of fluorescent lifetime imaging. Biophys J. 2010; 98:911–921.
Article11. Celli A, Mackenzie DS, Crumrine DS, Tu CL, Hupe M, Bikle DD, et al. Endoplasmic reticulum Ca2+ depletion activates XBP1 and controls terminal differentiation in keratinocytes and epidermis. Br J Dermatol. 2011; 164:16–25.
Article12. Celli A, Crumrine D, Meyer JM, Mauro TM. Endoplasmic reticulum calcium regulates epidermal barrier response and desmosomal structure. J Invest Dermatol. 2016; 136:1840–1847.
Article13. Kirschner N, Rosenthal R, Furuse M, Moll I, Fromm M, Brandner JM. Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J Invest Dermatol. 2013; 133:1161–1169.
Article14. Kurasawa M, Maeda T, Oba A, Yamamoto T, Sasaki H. Tight junction regulates epidermal calcium ion gradient and differentiation. Biochem Biophys Res Commun. 2011; 406:506–511.
Article15. Forslind B. Quantitative X-ray microanalysis of skin. Particle probe evaluation of the skin barrier function. Acta Derm Venereol Suppl (Stockh). 1987; 134:1–8.16. Feingold KR. Lamellar bodies: the key to cutaneous barrier function. J Invest Dermatol. 2012; 132:1951–1953.
Article17. Mao-Qiang M, Mauro T, Bench G, Warren R, Elias PM, Feingold KR. Calcium and potassium inhibit barrier recovery after disruption, independent of the type of insult in hairless mice. Exp Dermatol. 1997; 6:36–40.
Article18. Mauro T, Bench G, Sidderas-Haddad E, Feingold K, Elias P, Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J Invest Dermatol. 1998; 111:1198–1201.
Article19. Grubauer G, Elias PM, Feingold KR. Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res. 1989; 30:323–333.
Article20. Elias PM, Ahn SK, Denda M, Brown BE, Crumrine D, Kimutai LK, et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002; 119:1128–1136.
Article21. Choi EH, Kim MJ, Yeh BI, Ahn SK, Lee SH. Iontophoresis and sonophoresis stimulate epidermal cytokine expression at energies that do not provoke a barrier abnormality: lamellar body secretion and cytokine expression are linked to altered epidermal calcium levels. J Invest Dermatol. 2003; 121:1138–1144.
Article22. Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980; 19:245–254.
Article23. Pillai S, Bikle DD, Hincenbergs M, Elias PM. Biochemical and morphological characterization of growth and differentiation of normal human neonatal keratinocytes in a serum-free medium. J Cell Physiol. 1988; 134:229–237.
Article24. Eckert RL, Crish JF, Banks EB, Welter JF. The epidermis: genes on-genes off. J Invest Dermatol. 1997; 109:501–509.25. Ng DC, Shafaee S, Lee D, Bikle DD. Requirement of an AP-1 site in the calcium response region of the involucrin promoter. J Biol Chem. 2000; 275:24080–24088.
Article26. Presland RB, Bassuk JA, Kimball JR, Dale BA. Characterization of two distinct calcium-binding sites in the amino-terminus of human profilaggrin. J Invest Dermatol. 1995; 104:218–223.
Article27. Hitomi K. Transglutaminases in skin epidermis. Eur J Dermatol. 2005; 15:313–319.28. Lee YS, Dlugosz AA, McKay R, Dean NM, Yuspa SH. Definition by specific antisense oligonucleotides of a role for protein kinase C alpha in expression of differentiation markers in normal and neoplastic mouse epidermal keratinocytes. Mol Carcinog. 1997; 18:44–53.
Article29. Denning MF, Dlugosz AA, Williams EK, Szallasi Z, Blumberg PM, Yuspa SH. Specific protein kinase C isozymes mediate the induction of keratinocyte differentiation markers by calcium. Cell Growth Differ. 1995; 6:149–157.30. Deucher A, Efimova T, Eckert RL. Calcium-dependent involucrin expression is inversely regulated by protein kinase C (PKC)alpha and PKCdelta. J Biol Chem. 2002; 277:17032–17040.
Article31. Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004; 35:265–273.
Article32. Tu CL, Bikle DD. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pract Res Clin Endocrinol Metab. 2013; 27:415–427.
Article33. Oda Y, Tu CL, Pillai S, Bikle DD. The calcium sensing receptor and its alternatively spliced form in keratinocyte differentiation. J Biol Chem. 1998; 273:23344–23352.
Article34. Tu CL, Chang W, Bikle DD. The role of the calcium sensing receptor in regulating intracellular calcium handling in human epidermal keratinocytes. J Invest Dermatol. 2007; 127:1074–1083.
Article35. Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001; 276:41079–41085.
Article36. Blank JL, Ross AH, Exton JH. Purification and characterization of two G-proteins that activate the beta 1 isozyme of phosphoinositide-specific phospholipase C. Identification as members of the Gq class. J Biol Chem. 1991; 266:18206–18216.
Article37. Bikle DD, Ratnam A, Mauro T, Harris J, Pillai S. Changes in calcium responsiveness and handling during keratinocyte differentiation. Potential role of the calcium receptor. J Clin Invest. 1996; 97:1085–1093.
Article38. Filvaroff E, Calautti E, Reiss M, Dotto GP. Functional evidence for an extracellular calcium receptor mechanism triggering tyrosine kinase activation associated with mouse keratinocyte differentiation. J Biol Chem. 1994; 269:21735–21740.
Article39. Tu CL, Oda Y, Bikle DD. Effects of a calcium receptor activator on the cellular response to calcium in human keratinocytes. J Invest Dermatol. 1999; 113:340–345.
Article40. Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, et al. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol. 2012; 132:2350–2359.
Article41. Park K, Ikushiro H, Seo HS, Shin KO, Kim YI, Kim JY, et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc Natl Acad Sci U S A. 2016; 113:E1334–E1342.
Article42. Kim YI, Park K, Kim JY, Seo HS, Shin KO, Lee YM, et al. An endoplasmic reticulum stress-initiated sphingolipid metabolite, ceramide-1-phosphate, regulates epithelial innate immunity by stimulating β-defensin production. Mol Cell Biol. 2014; 34:4368–4378.
Article43. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012; 13:89–102.
Article44. Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007; 8:519–529.
Article45. Savignac M, Simon M, Edir A, Guibbal L, Hovnanian A. SERCA2 dysfunction in Darier disease causes endoplasmic reticulum stress and impaired cell-to-cell adhesion strength: rescue by Miglustat. J Invest Dermatol. 2014; 134:1961–1970.
Article46. Mauro T. Endoplasmic reticulum calcium, stress, and cell-to-cell adhesion. J Invest Dermatol. 2014; 134:1800–1801.
Article47. Graham DM, Huang L, Robinson KR, Messerli MA. Epidermal keratinocyte polarity and motility require Ca2+ influx through TRPV1. J Cell Sci. 2013; 126:4602–4613.
Article48. Tsai FC, Meyer T. Ca2+ pulses control local cycles of lamellipodia retraction and adhesion along the front of migrating cells. Curr Biol. 2012; 22:837–842.
Article49. Maroto R, Hamill OP. MscCa Regulation of tumor cell migration and metastasis. Curr Top Membr. 2007; 59:485–509.
Article50. Bourguignon LY, Ramez M, Gilad E, Singleton PA, Man MQ, Crumrine DA, et al. Hyaluronan-CD44 interaction stimulates keratinocyte differentiation, lamellar body formation/secretion, and permeability barrier homeostasis. J Invest Dermatol. 2006; 126:1356–1365.
Article51. Lee SE, Jun JE, Choi EH, Ahn SK, Lee SH. Stimulation of epidermal calcium gradient loss increases the expression of hyaluronan and CD44 in mouse skin. Clin Exp Dermatol. 2010; 35:650–657.
Article52. Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001; 24:487–517.
Article53. Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002; 9:229–231.
Article54. Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006; 68:619–647.
Article55. Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel). 2016; 9:E77.
Article56. Tóth BI, Oláh A, Szöllősi AG, Bíró T. TRP channels in the skin. Br J Pharmacol. 2014; 171:2568–2581.
Article57. Ho JC, Lee CH. TRP channels in skin: from physiological implications to clinical significances. Biophysics (Nagoya-shi). 2015; 11:17–24.
Article58. Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007; 87:165–217.
Article59. Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc. 2011; 15:33–39.
Article60. Voets T. Quantifying and modeling the temperaturedependent gating of TRP channels. Rev Physiol Biochem Pharmacol. 2012; 162:91–119.
Article61. Radtke C, Sinis N, Sauter M, Jahn S, Kraushaar U, Guenther E, et al. TRPV channel expression in human skin and possible role in thermally induced cell death. J Burn Care Res. 2011; 32:150–159.
Article62. Denda M, Fuziwara S, Inoue K, Denda S, Akamatsu H, Tomitaka A, et al. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem Biophys Res Commun. 2001; 285:1250–1252.
Article63. Tóth BI, Dobrosi N, Dajnoki A, Czifra G, Oláh A, Szöllosi AG, et al. Endocannabinoids modulate human epidermal keratinocyte proliferation and survival via the sequential engagement of cannabinoid receptor-1 and transient receptor potential vanilloid-1. J Invest Dermatol. 2011; 131:1095–1104.
Article64. Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science. 2002; 296:2046–2049.
Article65. Steinhoff M, Bíró T. A TR(I)P to pruritus research: role of TRPV3 in inflammation and itch. J Invest Dermatol. 2009; 129:531–535.
Article66. Nilius B, Bíró T. TRPV3: a ‘more than skinny’ channel. Exp Dermatol. 2013; 22:447–452.
Article67. Nilius B, Bíró T, Owsianik G. TRPV3: time to decipher a poorly understood family member. J Physiol. 2014; 592:295–304.
Article68. Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell. 2010; 141:331–343.
Article69. Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol. 2009; 129:714–722.
Article70. Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet. 2012; 90:558–564.
Article71. Lai-Cheong JE, Sethuraman G, Ramam M, Stone K, Simpson MA, McGrath JA. Recurrent heterozygous missense mutation, p.Gly573Ser, in the TRPV3 gene in an Indian boy with sporadic Olmsted syndrome. Br J Dermatol. 2012; 167:440–442.
Article72. Lehen'kyi V, Beck B, Polakowska R, Charveron M, Bordat P, Skryma R, et al. TRPV6 is a Ca2+ entry channel essential for Ca2+-induced differentiation of human keratinocytes. J Biol Chem. 2007; 282:22582–22591.73. Bianco SD, Peng JB, Takanaga H, Suzuki Y, Crescenzi A, Kos CH, et al. Marked disturbance of calcium homeostasis in mice with targeted disruption of the Trpv6 calcium channel gene. J Bone Miner Res. 2007; 22:274–285.
Article74. Montell C. TRP channels: mediators of sensory signaling and roles in health and disease. Chem Senses. 2006; 31:A45–A45.75. Liao Y, Plummer NW, George MD, Abramowitz J, Zhu MX, Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci U S A. 2009; 106:3202–3206.
Article76. Beck B, Lehen'kyi V, Roudbaraki M, Flourakis M, Charveron M, Bordat P, et al. TRPC channels determine human keratinocyte differentiation: new insight into basal cell carcinoma. Cell Calcium. 2008; 43:492–505.
Article77. Fatherazi S, Presland RB, Belton CM, Goodwin P, Al-Qutub M, Trbic Z, et al. Evidence that TRPC4 supports the calcium selective I(CRAC)-like current in human gingival keratinocytes. Pflugers Arch. 2007; 453:879–889.
Article78. Cai S, Fatherazi S, Presland RB, Belton CM, Roberts FA, Goodwin PC, et al. Evidence that TRPC1 contributes to calcium-induced differentiation of human keratinocytes. Pflugers Arch. 2006; 452:43–52.
Article79. Müller M, Essin K, Hill K, Beschmann H, Rubant S, Schempp CM, et al. Specific TRPC6 channel activation, a novel approach to stimulate keratinocyte differentiation. J Biol Chem. 2008; 283:33942–33954.
Article80. Leuner K, Kraus M, Woelfle U, Beschmann H, Harteneck C, Boehncke WH, et al. Reduced TRPC channel expression in psoriatic keratinocytes is associated with impaired differentiation and enhanced proliferation. PLoS One. 2011; 6:e14716.
Article81. Pani B, Cornatzer E, Cornatzer W, Shin DM, Pittelkow MR, Hovnanian A, et al. Up-regulation of transient receptor potential canonical 1 (TRPC1) following sarco(endo)plasmic reticulum Ca2+ ATPase 2 gene silencing promotes cell survival: a potential role for TRPC1 in Darier's disease. Mol Biol Cell. 2006; 17:4446–4458.
Article82. Denda M, Sokabe T, Fukumi-Tominaga T, Tominaga M. Effects of skin surface temperature on epidermal permeability barrier homeostasis. J Invest Dermatol. 2007; 127:654–659.
Article83. Yun JW, Seo JA, Jeong YS, Bae IH, Jang WH, Lee J, et al. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J Dermatol Sci. 2011; 62:8–15.
Article84. Sokabe T, Fukumi-Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010; 285:18749–18758.
Article85. Sokabe T, Tominaga M. The TRPV4 cation channel: a molecule linking skin temperature and barrier function. Commun Integr Biol. 2010; 3:619–621.86. Kida N, Sokabe T, Kashio M, Haruna K, Mizuno Y, Suga Y, et al. Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch. 2012; 463:715–725.
Article87. Akazawa Y, Yuki T, Yoshida H, Sugiyama Y, Inoue S. Activation of TRPV4 strengthens the tight-junction barrier in human epidermal keratinocytes. Skin Pharmacol Physiol. 2013; 26:15–21.
Article88. Denda M, Tsutsumi M, Goto M, Ikeyama K, Denda S. Topical application of TRPA1 agonists and brief cold exposure accelerate skin permeability barrier recovery. J Invest Dermatol. 2010; 130:1942–1945.
Article89. Denda M, Tsutsumi M, Denda S. Topical application of TRPM8 agonists accelerates skin permeability barrier recovery and reduces epidermal proliferation induced by barrier insult: role of cold-sensitive TRP receptors in epidermal permeability barrier homoeostasis. Exp Dermatol. 2010; 19:791–795.
Article90. Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009; 458:1093–1102.
Article91. Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol. 2006; 126:2664–2672.
Article92. Yamamoto-Kasai E, Yasui K, Shichijo M, Sakata T, Yoshioka T. Impact of TRPV3 on the development of allergic dermatitis as a dendritic cell modulator. Exp Dermatol. 2013; 22:820–824.
Article93. Huang SM, Lee H, Chung MK, Park U, Yu YY, Bradshaw HB, et al. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J Neurosci. 2008; 28:13727–13737.
Article94. Luo J, Feng J, Yu G, Yang P, Mack MR, Du J, et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J Allergy Clin Immunol. 2018; 141:608–619.
Article95. Vandenberghe M, Raphaël M, Lehen'kyi V, Gordienko D, Hastie R, Oddos T, et al. ORAI1 calcium channel orchestrates skin homeostasis. Proc Natl Acad Sci U S A. 2013; 110:E4839–E4848.
Article96. Darbellay B, Barnes L, Boehncke WH, Saurat JH, Kaya G. Reversal of murine epidermal atrophy by topical modulation of calcium signaling. J Invest Dermatol. 2014; 134:1599–1608.
Article97. Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013; 155:285–295.
Article98. Kumamoto J, Goto M, Denda S, Nakatani M, Takasugi Y, Tsuchiya K, et al. External negative electric potential accelerates exocytosis of lamellar bodies in human skin ex vivo. Exp Dermatol. 2013; 22:421–423.
Article99. Denda M, Fujiwara S, Hibino T. Expression of voltage-gated calcium channel subunit alpha1C in epidermal keratinocytes and effects of agonist and antagonists of the channel on skin barrier homeostasis. Exp Dermatol. 2006; 15:455–460.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Strontium Ions on Epidermal Permeability Barrier
- The Effect of Cold on the Skin Barrier
- Acute Modulations in Stratum Corneum Permeability Barrier Function Affect Claudin Expression and Epidermal Tight Junction Function via Changes of Epidermal Calcium Gradient
- The Iontophoresis Effect on Recovery After Acute Epidemal Barrier Disruption
- Atopic Dermatitis and Epidermal Barrier