Ann Dermatol.
2010 Aug;22(3):255-261. 10.5021/ad.2010.22.3.255.
Enhancement of Keratinocyte Differentiation by Rose Absolute Oil
- Affiliations
-
- 1Department of Dermatology and Institute of Health Sciences, School of Medicine, Gyeongsang National University, Jinju, Korea.
- 2Department of Dermatology and Research Institute for Medical Sciences, School of Medicine, Chungnam National University, Daejeon, Korea. jhoon@cnu.ac.kr
- 3Department of Visual Design, Daeduk University, Daejeon, Korea.
- 4Department of Advanced Organic Materials Engineering, Chonbuk National University, Jeonju, Korea.
- KMID: 2265357
- DOI: http://doi.org/10.5021/ad.2010.22.3.255
Abstract
- BACKGROUND
Through differentiation processes, keratinocytes provide a physical barrier to our bodies and control skin features such as moisturization, wrinkles and pigmentation. Keratinocyte differentiation is disturbed in several skin diseases such as psoriasis and atopic dermatitis.
OBJECTIVE
The aim of this study is to evaluate the keratinocyte differentiation-enhancing effect of rose absolute oil (RAO).
METHODS
Primary cultured human normal keratinocytes were treated with RAO, and differentiation then checked by the expression of marker genes.
RESULTS
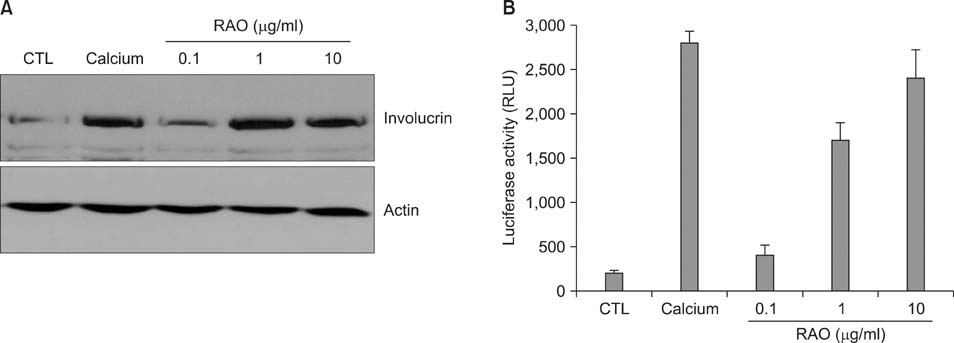
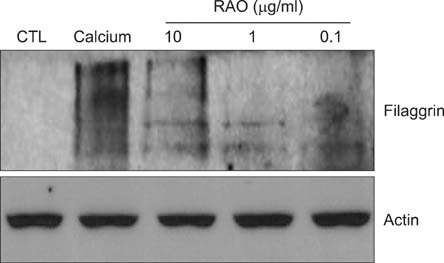
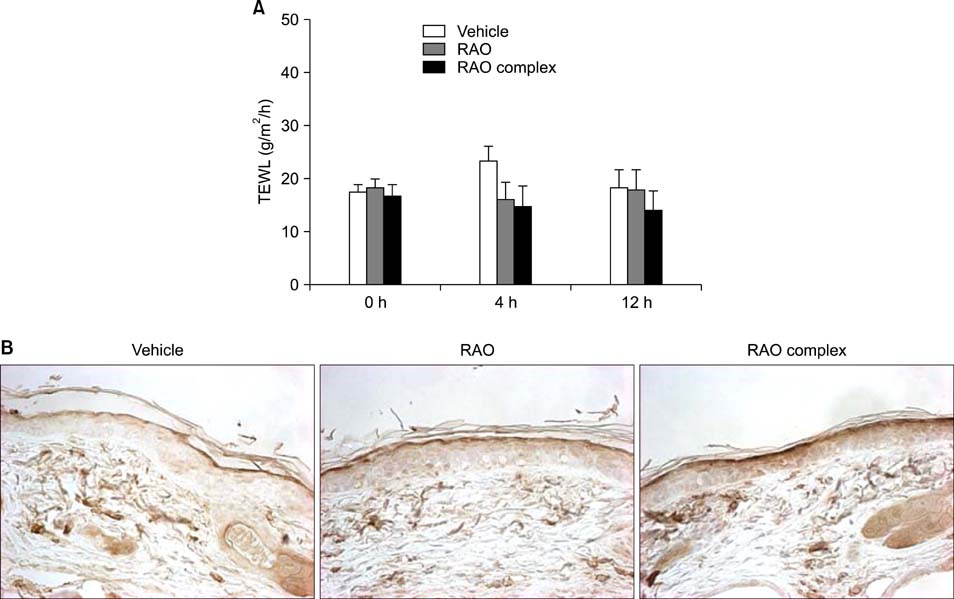
RAO did not induce cytotoxicity on cultured keratinocytes at a dose of 10microM. The level of involucrin, an early marker for keratinocyte differentiation, was significantly increased by RAO. Concomitantly, RAO increased involucrin promoter activity, indicating that RAO increased involucrin gene expression at the mRNA level. Furthermore, RAO increased the level of filaggrin in cultured keratinocytes, and in the granular layer of mouse skin. In line with these results, RAO decreased the proliferation of keratinocytes cultured in vitro. When RAO was applied topically on the tape-stripped mouse skins, it accelerated the recovery of disturbed barrier function.
CONCLUSION
These results suggest that RAO may be applicable for the control of skin texture and keratinocyte differentiation-related skin diseases.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Sox9 Increases the Proliferation and Colony-forming Activity of Outer Root Sheath Cells Cultured In Vitro
Ge Shi, Kyung-Cheol Sohn, Soo-Yeon Kim, Eun-Kyoung Ryu, Yeon-Suk Park, Young Lee, Young-Joon Seo, Jeung-Hoon Lee, Chang Deok Kim
Ann Dermatol. 2011;23(2):138-143. doi: 10.5021/ad.2011.23.2.138.
Reference
-
1. Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays. 2002. 24:789–800.
Article2. Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999. 31:5–19.
Article3. Blumenberg M, Tomić-Canić M. Human epidermal keratinocyte: keratinization processes. EXS. 1997. 78:1–29.
Article4. Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005. 125:183–200.
Article5. Toulza E, Mattiuzzo NR, Galliano MF, Jonca N, Dossat C, Jacob D, et al. Large-scale identification of human genes implicated in epidermal barrier function. Genome Biol. 2007. 8:R107.
Article6. Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin, and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995. 270:17702–17711.
Article7. Lowes MA, Bowcock AM, Krueger JG. Pathogenesis and therapy of psoriasis. Nature. 2007. 445:866–873.
Article8. Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, Fuentes-Duculan J, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009. 124:1235–1244.
Article9. Liu Y, Krueger JG, Bowcock AM. Psoriasis: genetic associations and immune system changes. Genes Immun. 2007. 8:1–12.
Article10. Lebwohl M, Herrmann LG. Impaired skin barrier function in dermatologic disease and repair with moisturization. Cutis. 2005. 76:6 Suppl. 7–12.11. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009. 361:496–509.
Article12. Lynde C. Moisturizers for the treatment of inflammatory skin conditions. J Drugs Dermatol. 2008. 7:1038–1043.13. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009. 84:1 Suppl. 2–15.14. Kezic S, Kammeyer A, Calkoen F, Fluhr JW, Bos JD. Natural moisturizing factor components in the stratum corneum as biomarkers of filaggrin genotype: evaluation of minimally invasive methods. Br J Dermatol. 2009. 161:1098–1104.
Article15. Kezic S, Kemperman PM, Koster ES, de Jongh CM, Thio HB, Campbell LE, et al. Loss-of-function mutations in the filaggrin gene lead to reduced level of natural moisturizing factor in the stratum corneum. J Invest Dermatol. 2008. 128:2117–2119.
Article16. Hwang C, Jang S, Choi DK, Kim S, Lee JH, Lee Y, et al. The role of Nkx2.5 in keratinocyte differentiation. Ann Dermatol. 2009. 21:376–381.
Article17. Sohn KC, Shi G, Jang S, Choi DK, Lee Y, Yoon TJ, et al. Pitx2, α β-catenin-regulated transcription factor, regulates the differentiation of outer root sheath cells cultured in vitro. J Dermatol Sci. 2009. 54:6–11.
Article18. Yoshizawa Y, Tanojo H, Kim SJ, Maibach HI. Sea water or its components alter experimental irritant dermatitis in man. Skin Res Technol. 2001. 7:36–39.
Article19. Seo EY, Namkung JH, Lee KM, Lee WH, Im M, Kee SH, et al. Analysis of calcium-inducible genes in keratinocytes using suppression subtractive hybridization and cDNA microarray. Genomics. 2005. 86:528–538.
Article20. Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001. 114(Pt 17):3069–3070.
Article21. Steinert PM, Marekov LN. Direct evidence that involucrin is a major early isopeptide cross-linked component of the keratinocyte cornified cell envelope. J Biol Chem. 1997. 272:2021–2030.
Article22. Robinson NA, Lapic S, Welter JF, Eckert RL. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J Biol Chem. 1997. 272:12035–12046.
Article23. Chu DH. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Development and structure of skin. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;57–73.24. Contet-Audonneau JL, Jeanmaire C, Pauly G. A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol. 1999. 140:1038–1047.
Article25. Elias PM, Menon G, Wetzel BK, Williams JJ. Evidence that stress to the epidermal barrier influenced the development of pigmentation in humans. Pigment Cell Melanoma Res. 2009. 22:420–434.
Article26. Kurokawa I, Mizutani H, Kusumoto K, Nishijima S, Tsujita-Kyutoku M, Shikata N, et al. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair Regen. 2006. 14:38–45.
Article27. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008. 17:1063–1072.
Article28. Ruether A, Stoll M, Schwarz T, Schreiber S, Fölster-Holst R. Filaggrin loss-of-function variant contributes to atopic dermatitis risk in the population of Northern Germany. Br J Dermatol. 2006. 155:1093–1094.
Article29. Proksch E, Fölster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci. 2006. 43:159–169.
Article30. Ulusoy S, Boşgelmez-Tinaz G, Secilmis-Canbay H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr Microbiol. 2009. 59:554–558.
Article31. Edlundh-Rose E, Kupershmidt I, Gustafsson AC, Parasassi T, Serafino A, Bracci-Laudiero L, et al. Gene expression analysis of human epidermal keratinocytes after N-acetyl L-cysteine treatment demonstrates cell cycle arrest and increased differentiation. Pathobiology. 2005. 72:203–212.
Article32. Balasubramanian S, Sturniolo MT, Dubyak GR, Eckert RL. Human epidermal keratinocytes undergo (-)-epigallocatechin-3-gallate-dependent differentiation but not apoptosis. Carcinogenesis. 2005. 26:1100–1108.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Single Low-Dose Radiation Induced Regulation of Keratinocyte Differentiation in Calcium-Induced HaCaT Cells
- The Role of Nkx2.5 in Keratinocyte Differentiation
- Stimulation of keratinocyte differentiation by extract of combined medicinal plant
- Effects of dietary fish oil on myocardical ischemia and reperfusion in isolated guinea pig heart
- Epigenetic Modulation of Gene Expression during Keratinocyte Differentiation