Korean J Urol.
2015 Sep;56(9):656-665. 10.4111/kju.2015.56.9.656.
Effect of curcumin on the interaction between androgen receptor and Wnt/beta-catenin in LNCaP xenografts
- Affiliations
-
- 1Department of Urology, Dankook University College of Medicine, Cheonan, Korea. hjh178@medimail.co.kr
- 2Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2344105
- DOI: http://doi.org/10.4111/kju.2015.56.9.656
Abstract
- PURPOSE
Curcumin is a nontoxic, chemopreventive agent possessing multifaceted functions. Our previous study showed that curcumin inhibits androgen receptor (AR) through modulation of Wnt/beta-catenin signaling in LNCaP cells. Therefore, we investigated the in vivo effects of curcumin by using LNCaP xenografts.
MATERIALS AND METHODS
LNCaP cells were subcutaneously inoculated in Balb/c nude mice. When the tumor volume reached greater than 100 mm3, either curcumin (500 mg/kg body weight) or vehicle was administered through oral gavage three times weekly for 4 weeks. The expression of AR and intermediate products of Wnt/beta-catenin were assessed.
RESULTS
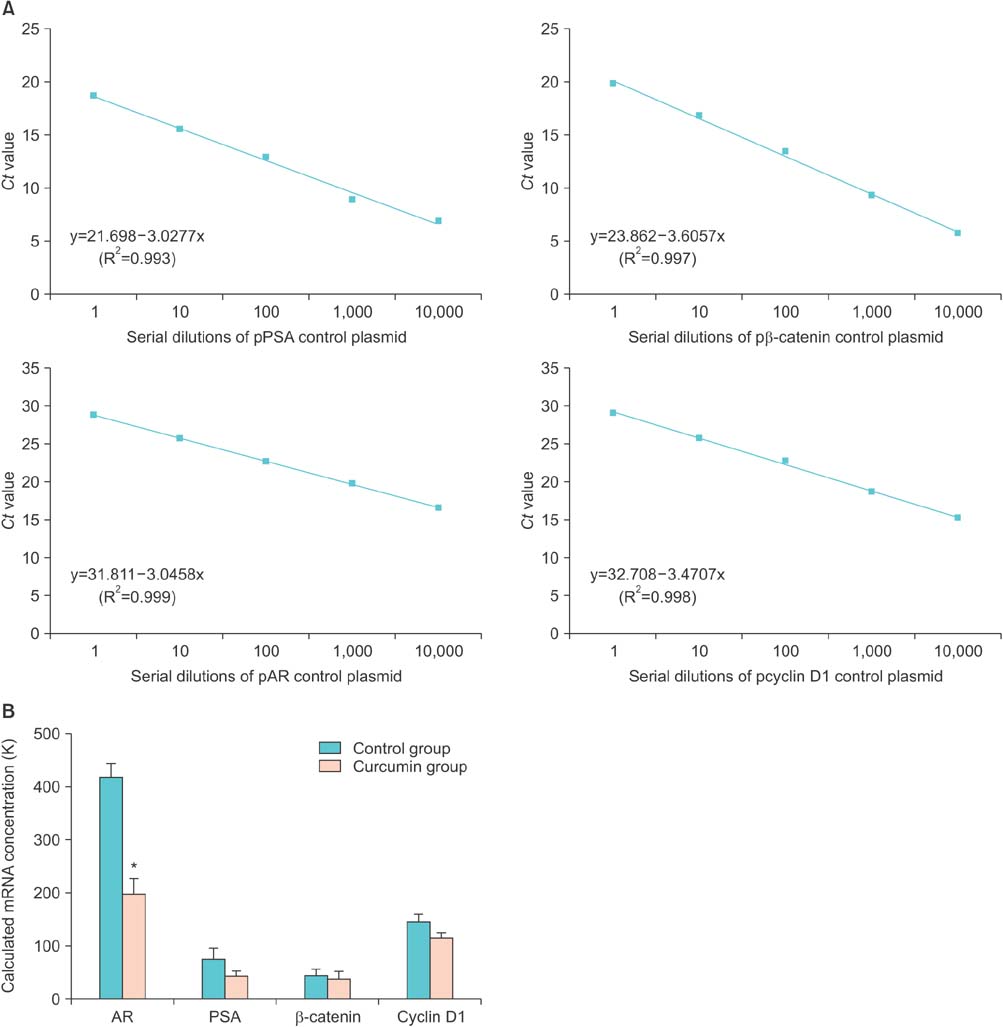
Curcumin had an inhibitory effect on tumor growth during the early period, which was followed by a slow increase in growth over time. Tumor growth was delayed about 27% in the curcumin group. The mean prostate-specific antigen (PSA) doubling time in the curcumin group was approximately twice that in the untreated group. Curcumin significantly decreased AR expression at both the mRNA and protein level. The PSA levels tended to be reduced in the curcumin group. However, there were no significant changes in expression of Wnt/beta-catenin pathway intermediates.
CONCLUSIONS
This study revealed that curcumin initially interferes with prostate cancer growth by inhibiting AR activity and possibly by reducing PSA expression. Further research is needed to investigate the plausible mechanism of the antiandrogenic action of curcumin.
MeSH Terms
-
Adenocarcinoma/drug therapy/*metabolism
Animals
Antineoplastic Agents/*pharmacology
Curcumin/*pharmacology
Cyclin D1/genetics/metabolism
Heterografts
Humans
Male
Mice, Inbred BALB C
Prostate-Specific Antigen/blood/genetics
Prostatic Neoplasms/drug therapy/*metabolism
RNA, Messenger/*metabolism
Receptors, Androgen/genetics/*metabolism
Wnt Signaling Pathway/*drug effects
beta Catenin/genetics/metabolism
Antineoplastic Agents
beta Catenin
Curcumin
Cyclin D1
RNA, Messenger
Receptors, Androgen
Prostate-Specific Antigen
Figure
Reference
-
1. Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004; 25:276–308.2. Richter E, Srivastava S, Dobi A. Androgen receptor and prostate cancer. Prostate Cancer Prostatic Dis. 2007; 10:114–118.3. Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002; 277:11336–11344.4. Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006; 99:402–410.5. Yang X, Chen MW, Terry S, Vacherot F, Bemis DL, Capodice J, et al. Complex regulation of human androgen receptor expression by Wnt signaling in prostate cancer cells. Oncogene. 2006; 25:3436–3444.6. Chesire DR, Isaacs WB. Beta-catenin signaling in prostate cancer: an early perspective. Endocr Relat Cancer. 2003; 10:537–560.7. Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005; 8:119–126.8. Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006; 237:22–32.9. Whitaker HC, Girling J, Warren AY, Leung H, Mills IG, Neal DE. Alterations in beta-catenin expression and localization in prostate cancer. Prostate. 2008; 68:1196–1205.10. Kypta RM, Waxman J. Wnt/β-catenin signalling in prostate cancer. Nat Rev Urol. 2012; 9:418–428.11. Horie S. Chemoprevention of prostate cancer: soy isoflavones and curcumin. Korean J Urol. 2012; 53:665–672.12. Duvoix A, Blasius R, Delhalle S, Schnekenburger M, Morceau F, Henry E, et al. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005; 223:181–190.13. Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008; 65:1631–1652.14. Oyagbemi AA, Saba AB, Ibraheem AO. Curcumin: from food spice to cancer prevention. Asian Pac J Cancer Prev. 2009; 10:963–967.15. Hutchins-Wolfbrandt A, Mistry AM. Dietary turmeric potentially reduces the risk of cancer. Asian Pac J Cancer Prev. 2011; 12:3169–3173.16. Nakamura K, Yasunaga Y, Segawa T, Ko D, Moul JW, Srivastava S, et al. Curcumin down-regulates AR gene expression and activation in prostate cancer cell lines. Int J Oncol. 2002; 21:825–830.17. Tsui KH, Feng TH, Lin CM, Chang PL, Juang HH. Curcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. J Androl. 2008; 29:661–668.18. Choi HY, Lim JE, Hong JH. Curcumin interrupts the interaction between the androgen receptor and Wnt/β-catenin signaling pathway in LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2010; 13:343–349.19. Sato N, Gleave ME, Bruchovsky N, Rennie PS, Beraldi E, Sullivan LD. A metastatic and androgen-sensitive human prostate cancer model using intraprostatic inoculation of LNCaP cells in SCID mice. Cancer Res. 1997; 57:1584–1589.20. Reynolds CP, Sun BC, DeClerck YA, Moats RA. Assessing growth and response to therapy in murine tumor models. Methods Mol Med. 2005; 111:335–350.21. Kyung YS, Park HY, Lee G. Preservation of uroplakins by 2-mercaptoethanesulfonate in cyclophosphamide-induced rat cystitis. Arch Toxicol. 2011; 85:51–57.22. Patel A, Dorey F, Franklin J, deKernion JB. Recurrence patterns after radical retropubic prostatectomy: clinical usefulness of prostate specific antigen doubling times and log slope prostate specific antigen. J Urol. 1997; 158:1441–1445.23. Maffezzini M, Bossi A, Collette L. Implications of prostate-specific antigen doubling time as indicator of failure after surgery or radiation therapy for prostate cancer. Eur Urol. 2007; 51:605–613.24. Bommareddy A, Eggleston W, Prelewicz S, Antal A, Witczak Z, McCune DF, et al. Chemoprevention of prostate cancer by major dietary phytochemicals. Anticancer Res. 2013; 33:4163–4174.25. Teiten MH, Gaascht F, Cronauer M, Henry E, Dicato M, Diederich M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int J Oncol. 2011; 38:603–611.26. Sundram V, Chauhan SC, Ebeling M, Jaggi M. Curcumin attenuates β-catenin signaling in prostate cancer cells through activation of protein kinase D1. PLoS One. 2012; 7:e35368.27. Hollingshead MG. Antitumor efficacy testing in rodents. J Natl Cancer Inst. 2008; 100:1500–1510.28. Perkins S, Verschoyle RD, Hill K, Parveen I, Threadgill MD, Sharma RA, et al. Chemopreventive efficacy and pharmaco-kinetics of curcumin in the min/+ mouse, a model of familial adenomatous polyposis. Cancer Epidemiol Biomarkers Prev. 2002; 11:535–540.29. Kurita T, Makino Y. Novel curcumin oral delivery systems. Anticancer Res. 2013; 33:2807–2821.30. Aras A, Khokhar AR, Qureshi MZ, Silva MF, Sobczak-Kupiec A, Pineda EA, et al. Targeting cancer with nano-bullets: curcumin, EGCG, resveratrol and quercetin on flying carpets. Asian Pac J Cancer Prev. 2014; 15:3865–3871.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Wnt/beta-Catenin Signalling and Androgen Receptor Expression in Prostate Cancer
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Regulation of Wnt signaling by protein-protein interaction and post-translational modifications
- Insulin-Like Growth Factor 1 Actions in Developing Brain and the Interaction with Wnt Pathway
- Effect of Decursin on the Expression of beta-Catenin and Matrix Metalloproteinase-7 in Prostate Cancer Cell Lines