World J Mens Health.
2013 Apr;31(1):36-46.
Clinical Significance of Wnt/beta-Catenin Signalling and Androgen Receptor Expression in Prostate Cancer
- Affiliations
-
- 1Department of Pathology, Inje University College of Medicine, Busan, Korea.
- 2Department of Advanced Fermentation Fusion Science & Technology, Kookmin University, Seoul, Korea.
- 3Section of Urologic Oncology and Dean and Betty Gallo Prostate Cancer Center, The Cancer Institute of New Jersey and Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
- 4Department of Urology, Inje University College of Medicine, Busan, Korea. urokang@lycos.co.kr
- 5Paik Institute of Clinical Research, Inje University College of Medicine, Busan, Korea.
- 6Department of Urology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
Abstract
- PURPOSE
To investigate the relationships among the Wnt/beta-catenin pathway, androgen receptor (AR), and clinicopathological factors in hormone-naive prostate cancer.
MATERIALS AND METHODS
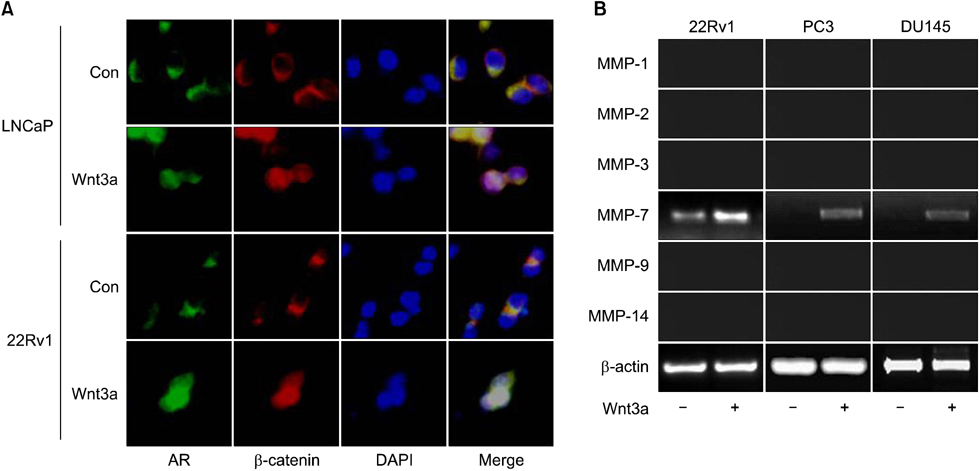
This study was conducted with132 cases of hormone-naive prostate cancer treated by prostatectomy and prostate needle biopsy. An immunohistochemical study using antibodies against beta-catenin, matrix metalloproteinase-7 (MMP-7), and the AR was performed. For the in vitro study, PC-3, LNCaP, 22Rv1, and DU145 cell lines were used.
RESULTS
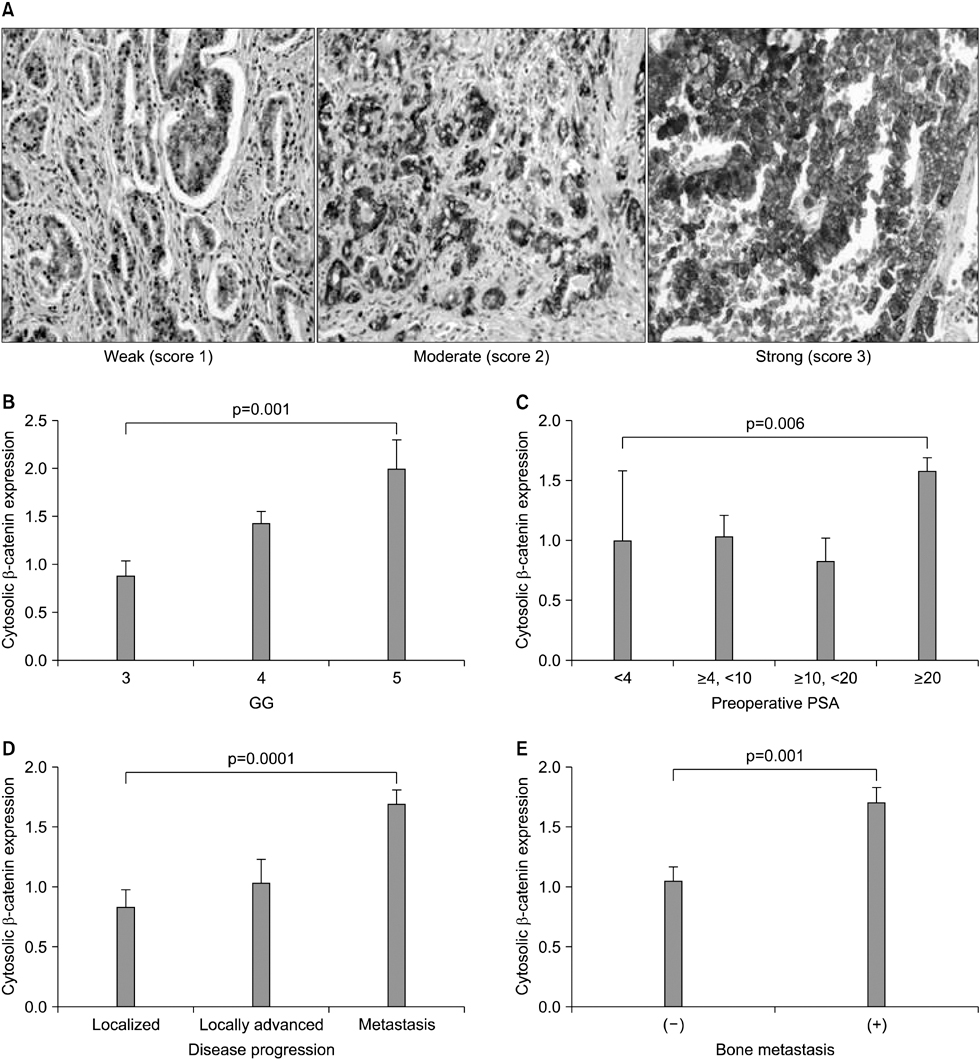
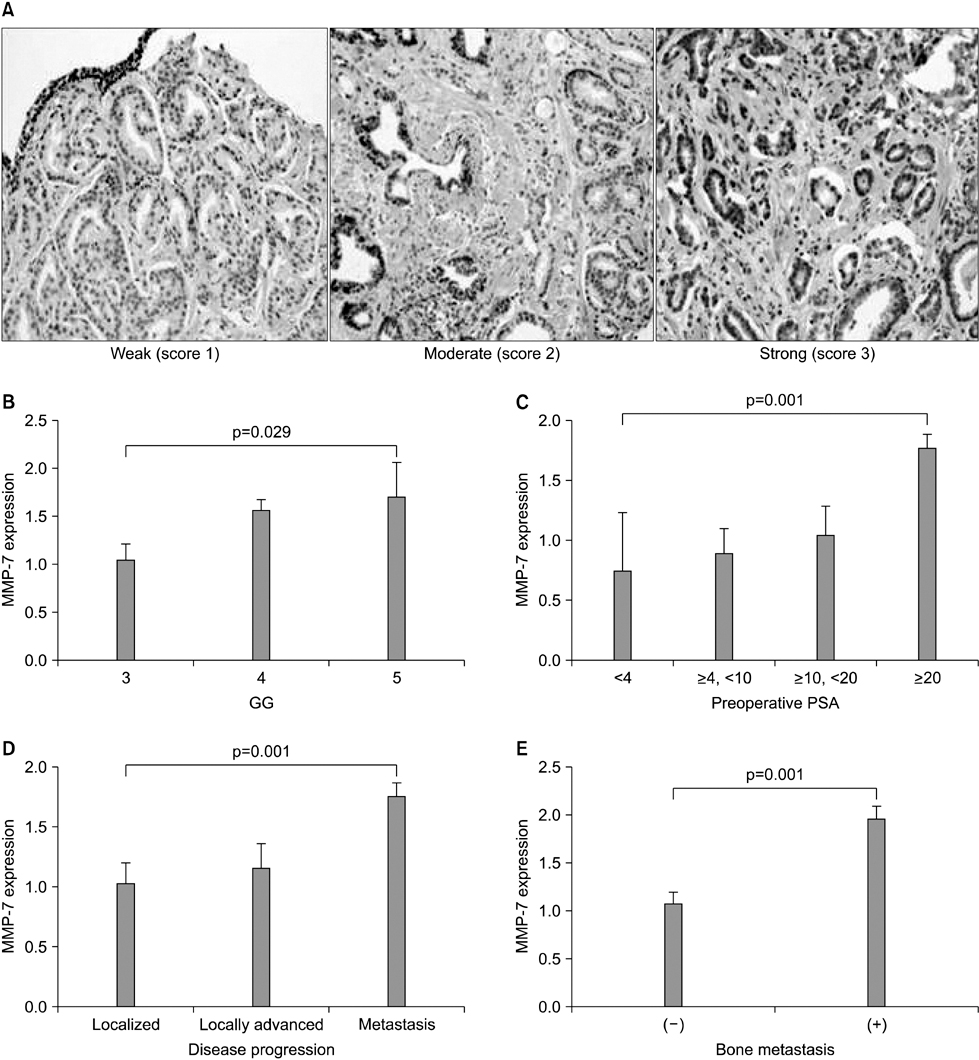
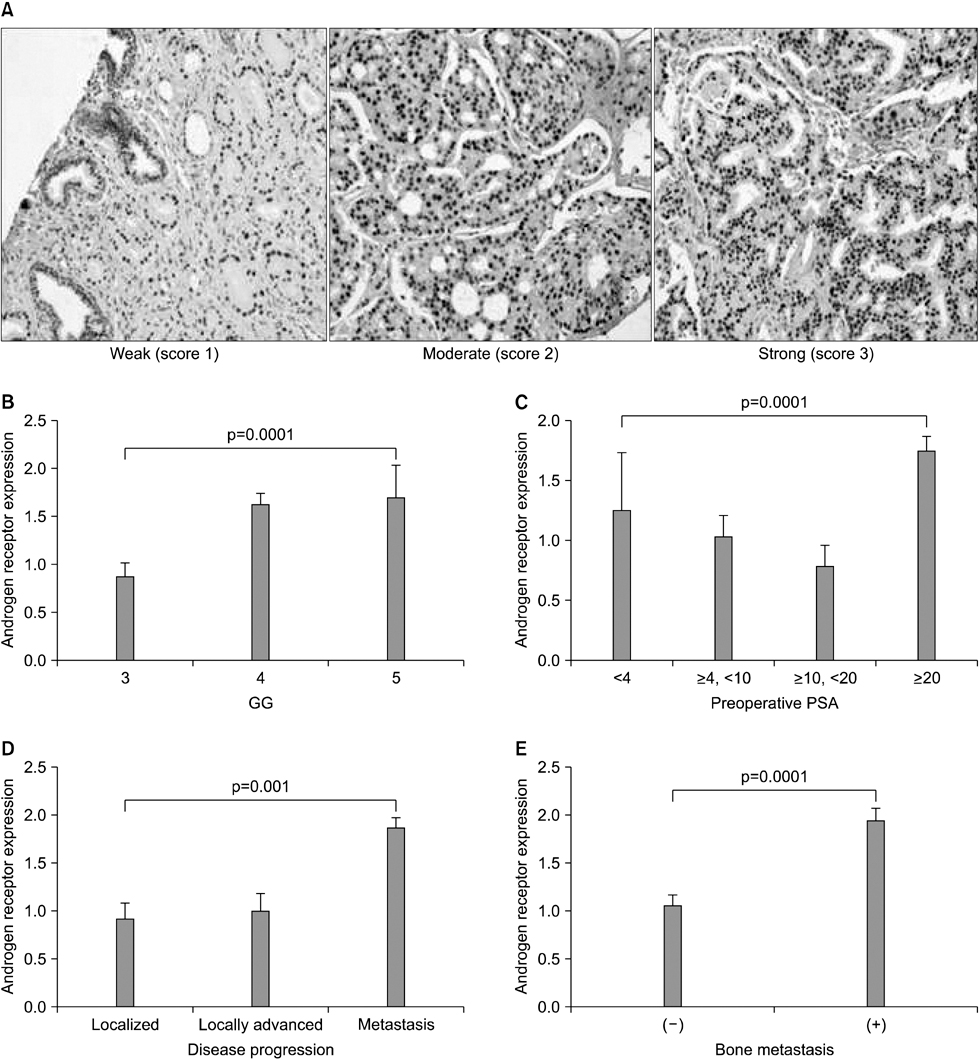
The clinical or pathological stage ware a localized cancer in 36 patients (27.3%), locally advanced cancer in 31 (23.5%), and metastatic cancer in 65 (49.2%). We detected increased beta-catenin, AR, and MMP-7 expression with a high Gleason grade, disease progression, and increasing serum prostate-specific antigen (PSA) levels (p<0.01). In Spearman's rank correlations, the expression of cytoplasmic beta-catenin, MMP-7, and the AR were found to be significantly positively correlated. In addition, the expression of beta-catenin, MMP-7, and the AR were significantly correlated with clinicopathological variables indicative of a poor prognosis. Forty-nine patients with primary androgen deprivation had short response durations from hormone therapy to PSA progression with elevated MMP-7 expression on the Kaplan-Meier curve (p=0.0036).
CONCLUSIONS
These data show that an activated Wnt/beta-catenin pathway and AR expression in prostate cancer are correlated with metastasis and aggressiveness. In addition, the expression of MMP-7 protein, a target of the Wnt/beta-catenin pathway, is associated with PSA progression in prostate cancer patients undergoing primary hormone therapy.
MeSH Terms
-
Antibodies
beta Catenin
Biopsy, Needle
Cell Line
Cytoplasm
Disease Progression
Humans
Matrix Metalloproteinase 7
Matrix Metalloproteinases
Neoplasm Metastasis
Prognosis
Prostate
Prostate-Specific Antigen
Prostatectomy
Prostatic Neoplasms
Receptors, Androgen
Antibodies
Matrix Metalloproteinase 7
Matrix Metalloproteinases
Prostate-Specific Antigen
Receptors, Androgen
beta Catenin
Figure
Reference
-
1. Wang G, Wang J, Sadar MD. Crosstalk between the androgen receptor and beta-catenin in castrate-resistant prostate cancer. Cancer Res. 2008. 68:9918–9927.2. Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999. 18:2883–2891.3. Saeki H, Tanaka S, Sugimachi K, Kimura Y, Miyazaki M, Ohga T, et al. Interrelation between expression of matrix metalloproteinase 7 and beta-catenin in esophageal cancer. Dig Dis Sci. 2002. 47:2738–2742.4. Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009. 19:683–697.
Article5. Schweizer L, Rizzo CA, Spires TE, Platero JS, Wu Q, Lin TA, et al. The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens. BMC Cell Biol. 2008. 9:4.
Article6. Li YJ, Wei ZM, Meng YX, Ji XR. Beta-catenin up-regulates the expression of cyclinD1, c-myc and MMP-7 in human pancreatic cancer: relationships with carcinogenesis and metastasis. World J Gastroenterol. 2005. 11:2117–2123.7. Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol. 1999. 155:1033–1038.8. Stewart AJ, Scher HI, Chen MH, McLeod DG, Carroll PR, Moul JW, et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol. 2005. 23:6556–6560.
Article9. Kwak C, Jeong SJ, Park MS, Lee E, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002. 168:995–1000.
Article10. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008. 26:1148–1159.
Article11. de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003. 9:1801–1807.12. Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004. 101:1345–1356.13. Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Invest. 2008. 88:1340–1348.14. Szarvas T, Becker M, vom Dorp F, Gethmann C, Tötsch M, Bánkfalvi A, et al. Matrix metalloproteinase-7 as a marker of metastasis and predictor of poor survival in bladder cancer. Cancer Sci. 2010. 101:1300–1308.
Article15. Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003. 36:128–137.
Article16. Robertson BW, Chellaiah MA. Osteopontin induces beta-catenin signaling through activation of Akt in prostate cancer cells. Exp Cell Res. 2010. 316:1–11.17. Maruta S, Sakai H, Kanda S, Hayashi T, Kanetake H, Miyata Y. E1AF expression is associated with extra-prostatic growth and matrix metalloproteinase-7 expression in prostate cancer. APMIS. 2009. 117:791–796.
Article18. McDonnell S, Navre M, Coffey RJ Jr, Matrisian LM. Expression and localization of the matrix metalloproteinase pump-1 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991. 4:527–533.
Article19. Hashimoto K, Kihira Y, Matuo Y, Usui T. Expression of matrix metalloproteinase-7 and tissue inhibitor of metalloproteinase-1 in human prostate. J Urol. 1998. 160:1872–1876.
Article20. Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, et al. Linking beta-catenin to androgen-signaling pathway. J Biol Chem. 2002. 277:11336–11344.21. Truica CI, Byers S, Gelmann EP. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000. 60:4709–4713.22. Boxler S, Djonov V, Kessler TM, Hlushchuk R, Bachmann LM, Held U, et al. Matrix metalloproteinases and angiogenic factors: predictors of survival after radical prostatectomy for clinically organ-confined prostate cancer? Am J Pathol. 2010. 177:2216–2224.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of curcumin on the interaction between androgen receptor and Wnt/beta-catenin in LNCaP xenografts
- Effect of Decursin on the Expression of beta-Catenin and Matrix Metalloproteinase-7 in Prostate Cancer Cell Lines
- Suppression of β-catenin Signaling Pathway in Human Prostate Cancer PC3 Cells by Delphinidin
- Expression of Wnt 1 and beta-catenin in epithelial ovarian cancer
- Expression of Beta-catenin and E-cadherin in Early Gastric Cancer: Correlation with Clinicopathologic Parameters