J Korean Soc Hypertens.
2012 Sep;18(3):126-135.
Redox Regulating Protein APE1/Ref-1 Expression is Increased in Abdominal Aortic Coarctation-induced Hypertension Rats
- Affiliations
-
- 1Department of Physiology, Chungnam National University School of Medicine, Daejeon, Korea. bhjeon@cnu.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Chungnam National University School of Medicine, Daejeon, Korea.
Abstract
- BACKGROUND
Aim of study is designed to investigate whether apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE1/Ref-1) expression is changed in abdominal aortic coarctation models.
METHODS
Male Sprague-Dawley rats were randomly assigned with abdominal aortic coarctation, repaired group, sham, and control groups. Endothelial function was assessed with endothelium-dependent relaxations. Detection of superoxide anion and lipid peroxidation was performed by lucigenin chemiluminescence and thiobarbituric acid-reactive substances assay. APE1/Ref-1 expression was measured with Western blot and immunohistochemistry.
RESULTS
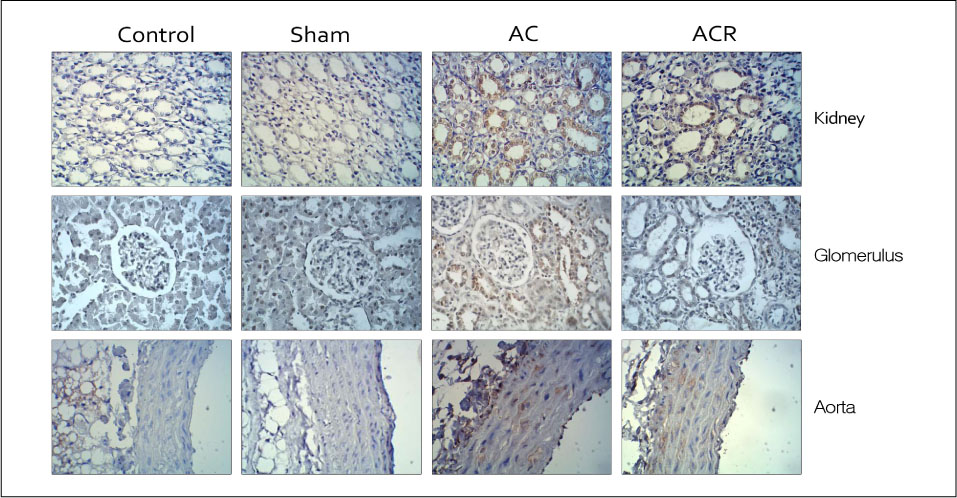
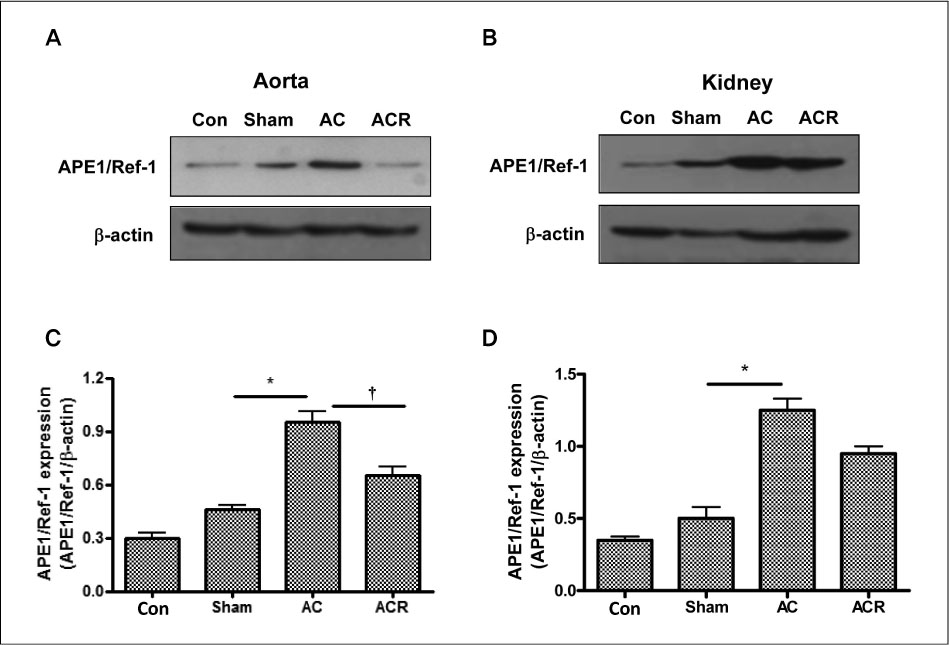
In anesthetized condition, the abdominal aortic coarctation rats showed hypertension as systolic/diastolic arterial pressure of 171/114 mm Hg, compared with 114/94 mm Hg of control. Endothelium-dependent relaxations were significantly impaired in the aortic coarctation which was recovered in 1 week after coarctation repair. Superoxide production and lipid peroxidation were elevated in aortic coarctation rats. In immunohistochemistry, APE1/Ref-1 expressions were increased at aorta and kidney in aortic coarctation rats. Increased APE1/Ref-1 expression in aorta was recovered by repair of coarctation.
CONCLUSIONS
Taken together, it suggests that APE1/Ref-1 expression was increased in aortic coarctation-induced hypertensive rats, suggesting a biomarker for hypertension. Impaired endothelium dependent relaxation in the aortic coarctation can be modulated by repair of coarctation or the modulation of blood pressure.
Keyword
MeSH Terms
-
Acridines
Animals
Aorta
Aortic Coarctation
Arterial Pressure
Blood Pressure
Blotting, Western
Endothelium
Humans
Hypertension
Immunohistochemistry
Kidney
Lipid Peroxidation
Luminescence
Male
Oxidation-Reduction
Oxidative Stress
Rats
Rats, Sprague-Dawley
Relaxation
Salicylamides
Superoxides
Acridines
Salicylamides
Superoxides
Figure
Reference
-
1. Demple B, Herman T, Chen DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991. 88:11450–11454.
Article2. Robson CN, Hickson ID. Isolation of cDNA clones encoding a human apurinic/apyrimidinic endonuclease that corrects DNA repair and mutagenesis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991. 19:5519–5523.3. Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009. 11:601–620.
Article4. Lee HM, Yuk JM, Shin DM, Yang CS, Kim KK, Choi DK, et al. Apurinic/apyrimidinic endonuclease 1 is a key modulator of keratinocyte inflammatory responses. J Immunol. 2009. 183:6839–6848.
Article5. Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol. 2005. 25:29–38.
Article6. Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000. 36:142–146.
Article7. Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1994. 74:139–162.
Article8. Fernandes M, Onesti G, Weder A, Dykyj R, Gould AB, Kim KE, et al. Experimental model of severe renal hypertension. J Lab Clin Med. 1976. 87:561–567.9. Parker FB Jr, Streeten DH, Farrell B, Blackman MS, Sondheimer HM, Anderson GH Jr. Preoperative and postoperative renin levels in coarctation of the aorta. Circulation. 1982. 66:513–514.
Article10. Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, et al. Apurinic/apyrimidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004. 95:902–910.11. Sengupta S, Chattopadhyay R, Mantha AK, Mitra S, Bhakat KK. Regulation of mouse-renin gene by apurinic/apyrimidinic-endonuclease 1 (APE1/Ref-1) via recruitment of histone deacetylase 1 corepressor complex. J Hypertens. 2012. 30:917–925.
Article12. Naganuma T, Nakayama T, Sato N, Fu Z, Soma M, Yamaguchi M, et al. Haplotype-based case-control study on human apurinic/apyrimidinic endonuclease 1/redox effector factor-1 gene and essential hypertension. Am J Hypertens. 2010. 23:186–191.
Article13. Rojo-Ortega JM, Genest J. A method for production of experimental hypertension in rats. Can J Physiol Pharmacol. 1968. 46:883–885.
Article14. Lee SK, Kim HS, Song YJ, Joo HK, Lee JY, Lee KH, et al. Alteration of p66shc is associated with endothelial dysfunction in the abdominal aortic coarctation of rats. FEBS Lett. 2008. 582:2561–2566.
Article15. Yoo DG, Song YJ, Cho EJ, Lee SK, Park JB, Yu JH, et al. Alteration of APE1/ref-1 expression in non-small cell lung cancer: the implications of impaired extracellular superoxide dismutase and catalase antioxidant systems. Lung Cancer. 2008. 60:277–284.
Article16. Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW, Jeon BH. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin. 2002. 23:1152–1156.17. Lee SK, Chung JI, Park MS, Joo HK, Lee EJ, Cho EJ, et al. Apurinic/apyrimidinic endonuclease 1 inhibits protein kinase C-mediated p66shc phosphorylation and vasoconstriction. Cardiovasc Res. 2011. 91:502–509.
Article18. Yayama K, Horii M, Hiyoshi H, Takano M, Okamoto H, Kagota S, et al. Up-regulation of angiotensin II type 2 receptor in rat thoracic aorta by pressure-overload. J Pharmacol Exp Ther. 2004. 308:736–743.
Article19. Goetz RM, Holtz J. Enhanced angiotensin-converting enzyme activity and impaired endothelium-dependent vasodilation in aortae from hypertensive rats: evidence for a causal link. Clin Sci (Lond). 1999. 97:165–174.
Article20. Bell DR. Vascular smooth muscle responses to endothelial autacoids in rats with chronic coarctation hypertension. J Hypertens. 1993. 11:65–74.
Article21. Ishimitsu T. Antihypertensive therapy considering the prevention of vascular aging. J Korean Soc Hypertens. 2011. 17:85–94.
Article22. Choo EH, Ihm SH, Kim OR, Jang SW, Park CS, Kim HY, et al. Imatinib mesylate attenuates cardiac fibrosis in spontaneously hypertensive rats. J Korean Soc Hypertens. 2011. 17:48–56.
Article23. Touyz RM. Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep. 2000. 2:98–105.
Article24. Sindhu RK, Roberts CK, Ehdaie A, Zhan CD, Vaziri ND. Effects of aortic coarctation on aortic antioxidant enzymes and NADPH oxidase protein expression. Life Sci. 2005. 76:945–953.
Article25. Vaziri ND, Ni Z. Expression of NOX-I, gp91phox, p47phox and P67phox in the aorta segments above and below coarctation. Biochim Biophys Acta. 2005. 1723:321–327.
Article26. Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol. 2004. 165:219–226.27. Jeon BH, Irani K. APE1/Ref-1: versatility in progress. Antioxid Redox Signal. 2009. 11:571–574.
Article28. Ramana CV, Boldogh I, Izumi T, Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci U S A. 1998. 95:5061–5066.
Article29. Grosch S, Fritz G, Kaina B. Apurinic endonuclease (Ref-1) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Res. 1998. 58:4410–4416.30. Bhakat KK, Mantha AK, Mitra S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid Redox Signal. 2009. 11:621–638.
Article31. Grosch S, Kaina B. Transcriptional activation of apurinic/apyrimidinic endonuclease (Ape, Ref-1) by oxidative stress requires CREB. Biochem Biophys Res Commun. 1999. 261:859–863.32. Harrison L, Ascione AG, Takiguchi Y, Wilson DM 3rd, Chen DJ, Demple B. Comparison of the promoters of the mouse (APEX) and human (APE) apurinic endonuclease genes. Mutat Res. 1997. 385:159–172.
Article33. Rose P, Bond J, Tighe S, Toth MJ, Wellman TL, Briso de Montiano EM, et al. Genes overexpressed in cerebral arteries following salt-induced hypertensive disease are regulated by angiotensin II, JunB, and CREB. Am J Physiol Heart Circ Physiol. 2008. 294:H1075–H1085.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Altered Secretory Activity of APE1/Ref-1 D148E Variants Identified in Human Patients With Bladder Cancer
- Dynamic Regulation of APE1/Ref-1 as a Therapeutic Target Protein
- APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions
- Ape1/Ref-1 Stimulates GDNF/GFR alpha1-mediated Downstream Signaling and Neuroblastoma Proliferation
- APE1/Ref-1 as a Serological Biomarker for the Detection of Bladder Cancer