J Korean Assoc Oral Maxillofac Surg.
2010 Oct;36(5):346-352.
The effect of heat shock protein 70 on inducible nitric oxide synthase during sepsis in rats
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Institute of Oral Bioscience, Brain Korea 21 project, Chonbuk National University, Jeonju, Korea. hkshin@jbnu.ac.kr
Abstract
- INTRODUCTION
Heat shock protein70 (HSP70) is a highly conserved family of proteins produced after a variety of stresses. Many studies reported that the overexpression of HSP70 can improve the prognosis of the patients with sepsis through a reduction of the nitric oxide concentration. However, these results only revealed the effect of HSP70 and nitric oxide. No studies have examined the relationship between HSP70 and nitric oxide. The aim of this study was to evaluate the effect of the overexpression of HSP70 on the expression of inducible nitric oxide synthase and the nitric oxide concentration. In addition, the mechanism of the relationship of HSP70 and inducible nitric oxide synthase (iNOS) in sepsis was examined.
MATERIALS AND METHODS
The experiments were performed on male sprague-dawley rats. Sepsis was induced by a cecal ligation and puncture (CLP). Glutamine (GLN) or saline was administered 1 hour after the initiation of sepsis. Serum and lung tissues were acquired from the rats 12 hours or 24 hours after the initiation of sepsis. The nitric oxide concentration, the expression of HSP70 in lung, and the gene expression of iNOS in lung were analyzed. The three groups, sham operation, CLP and CLP+GLN, were compared.
RESULTS
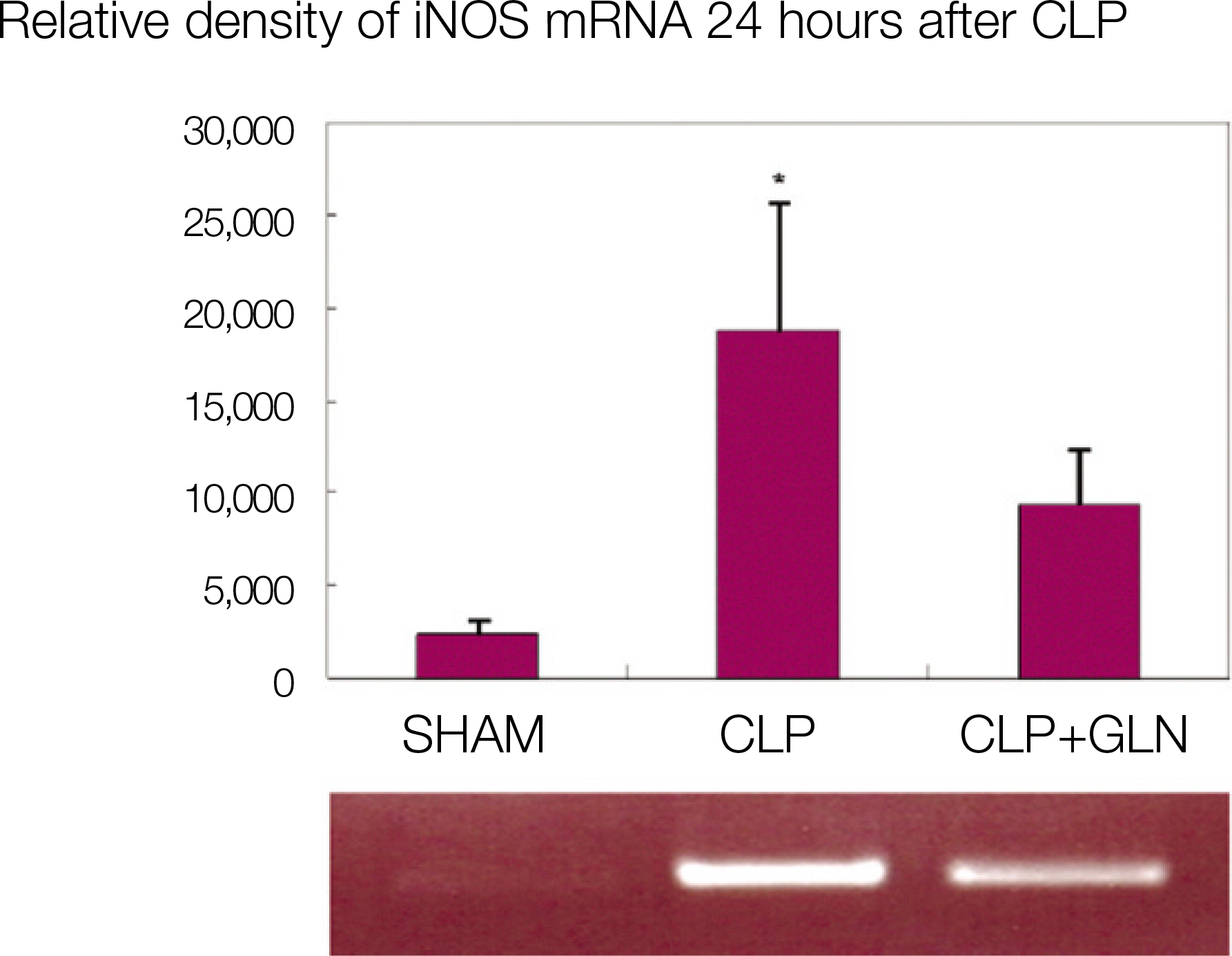
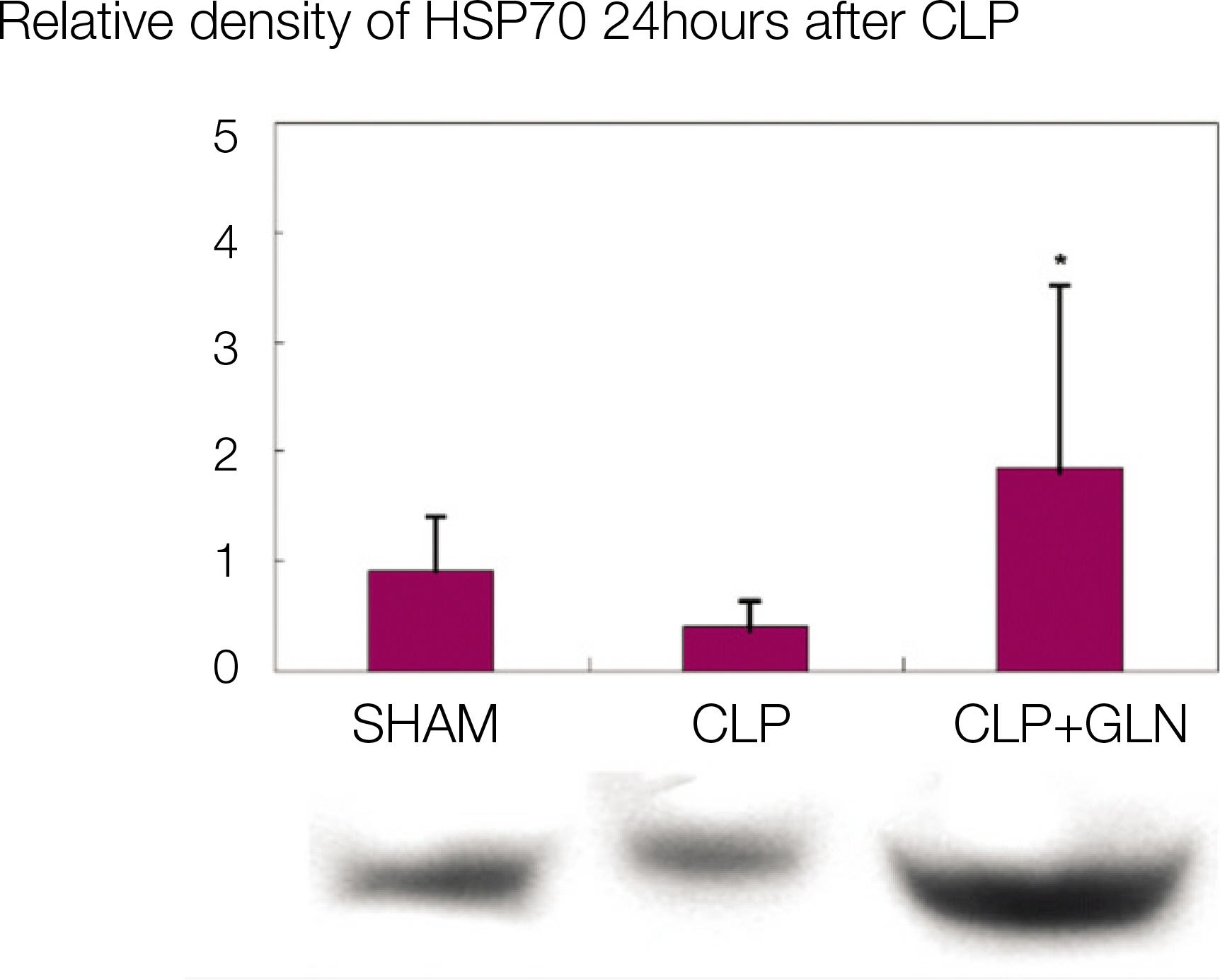
Compared to the other groups, in CLP+GLN, GLN administered after the initiation of sepsis enhanced the expression of HSP70 in the lung at 12 hours (47.19+/-10.04 vs. 33.22+/-8.28, P=0.025) and 24 hours (47.06+/-10.60 vs. 31.90+/-4.83, P=0.004). In CLP+GLN, GLN attenuated the expression of iNOS messenger RNA (mRNA) in the lung at 12 hours (5,513.73+/-1,051.60 vs. 4,167.17+/-951.59, P=0.025) and 24 hours (18,740.27 +/-8,241.20 vs. 9,437.65+/-2,521.07, P=0.016), and reduced the concentration of nitric oxide in the serum at 12 hours (0.86+/-0.48 vs. 3.82+/-2.53, P=0.016) and 24 hours (0.39+/-0.25 vs. 1.85+/-1.70, P=0.025).
CONCLUSION
The overexpression of HSP70 induced by the administration of GLN in sepsis attenuates the expression of the iNOS gene but reduces the nitric oxide concentration.
MeSH Terms
-
Animals
Gene Expression
Glutamine
Heat-Shock Proteins
Hot Temperature
HSP70 Heat-Shock Proteins
Humans
Ligation
Lung
Male
Nitric Oxide
Nitric Oxide Synthase
Nitric Oxide Synthase Type II
Prognosis
Proteins
Punctures
Rats
Rats, Sprague-Dawley
RNA, Messenger
Salicylamides
Sepsis
Shock
Glutamine
HSP70 Heat-Shock Proteins
Heat-Shock Proteins
Nitric Oxide
Nitric Oxide Synthase
Nitric Oxide Synthase Type II
Proteins
RNA, Messenger
Salicylamides
Figure
Reference
-
References
1. Dong HP, Chen HW, Hsu C, Chiu HY, Lin LC, Yang RC. Previous heat shock treatment attenuates lipopolysaccharide-induced hyporesponsiveness of platelets in rats. Shock. 2005; 24:239–44.
Article2. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29:1303–10.
Article3. Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001; 29(7 Suppl):S109–16.
Article4. O'Brien JM Jr, Abraham E. New approaches to the treatment of sepsis. Clin Chest Med. 2003; 24:521–48.5. Dellinger RP. Cardiovascular management of septic shock. Crit Care Med. 2003; 31:946–55.
Article6. Holmes CL, Russell JA, Walley KR. Genetic polymorphisms in sepsis and septic shock: role in prognosis and potential for therapy. Chest. 2003; 124:1103–15.7. Chen HW, Hsu C, Lu TS, Wang SJ, Yang RC. Heat shock pretreatment prevents cardiac mitochondrial dysfunction during sepsis. Shock. 2003; 20:274–9.
Article8. Pinsky MR. Dysregulation of the immune response in severe sepsis. Am J Med Sci. 2004; 328:220–9.
Article9. Cohen RI, Wilson D, Liu SF. Nitric oxide modifies the sarcoplasmic reticular calcium release channel in endotoxemia by both guanosine-3', 5'(cyclic) phosphate-dependent and independent pathways. Crit Care Med. 2006; 34:173–81.10. Cariou A, Vinsonneau C, Dhainaut JF. Adjunctive therapies in sepsis: an evidence-based review. Crit Care Med. 2004; 32(11 Suppl):S562–70.
Article11. Opal SM, Glu ¨ck T. Endotoxin as a drug target. Crit Care Med. 2003; 31(1 Suppl):S57–64.
Article12. Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Aantagonist Sepsis Investigator Group. Crit Care Med. 1997; 25:1115–24.13. Wenzel RP. Treating sepsis. N Engl J Med. 2002; 347:966–7.
Article14. Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999; 340:207–14.
Article15. Annane D, Se ′ bille V, Charpentier C, Bollaert PE, Franc¸ois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fluorocortisone on mortality in patients with septic shock. JAMA. 2002; 288:862–71.16. Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001; 344:699–709.
Article17. Corrigan JJ Jr. Heparin therapy in bacterial septicemia. J Pediatr. 1977; 91:695–700.
Article18. Abraham E, Anzueto A, Gutierrez G, Tessler S, San Pedro G, Wunderink R, et al. Double-blind randomized controlled trial of monoclonal antibody to human tumour necrosis factor in treatment of septic shock. NORASEPT II Study Group. Lancet. 1998; 351:929–33.19. Cole L, Bellomo R, Hart G, Journois D, Davenport P, Tipping P, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002; 30:100–6.
Article20. Sander A, Armbruster W, Sander B, Daul AE, Lange R, Peters J. Hemofiltration increases IL-6 clearance in early systemic inflammatory response syndrome but dose not alter IL-6 and TNF-α plasma concentrations. Intensive Care Med. 1997; 23:878–84.21. Kellum JA, Johnson JP, Kramer D, Palevsky P, Brady JJ, Pinsky MR. Diffusive vs. convective therapy: effects on mediators of inflammation in patients with severe systemic inflammatory response syndrome. Crit Care Med. 1998; 26:1995–2000.22. Braun N, Rosenfeld S, Giolai M, Banzhaf W, Fretschner R, Warth H. Effects of continuous hemodiafiltration on IL-6, TNF-alpha, C3a and TCC in patients with SIRS/septic shock using two different membranes. Contrib Nephrol. 1995; 116:89–98.23. McMaster P, Shann F. The use of extracorporeal techniques to remove humoral factors in sepsis. Pediatr Crit Care Med. 2003; 4:2–7.
Article24. Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004; 32:858–73.
Article25. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001; 345:1368–77.
Article26. Rhodes A, Bennett ED. Early-goal directed therapy: an evidence-based review. Crit Care Med. 2004; 32(11 Suppl):S448–50.27. Thiemermann C, Szabo ′C, Mitchell JA, Vane JR. Vascular hy-poreactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc Natl Acad Sci U S A. 1993; 90:267–71.
Article28. Aranow JS, Zhuang J, Wang H, Larkin V, Smith M, Fink MP. A selective inhibitor of inducible in nitric oxide synthase prolongs survival in a rat model of bacterial peritonitis: comparison with two nonselective strategies. Shock. 1996; 5:116–21.29. Grover R, Zaccardelli D, Colice G, Guntupalli K, Watson D, Vincent JL. An open-label dose escalation study of the nitric oxide synthase inhibitor NG-methyl-arginine hydrochloride (546C88) in patients with septic shock. Crit Care Med. 1999; 27:913–22.30. Watson D, Grover R, Anzueto A, Lorente J, Smithies M, Bellomo R, et al. Cardiovascular effects of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) in patients with septic shock: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144–002). Crit Care Med. 2004; 32:13–20.
Article31. Ritossa FA. A new puffing pattern induced by temperature shock and DNP in Drosophillia. Experientia. 1962; 18:571–3.32. Tandara AA, Kloeters O, Kim I, Mogford JE, Mustoe TA. Age effect on HSP70: decreased resistance to ischemic and oxidative stress in HDF. J Surg Res. 2006; 132:32–9.
Article33. Sigal LH. Molecular biology and immunology for clinicians 18: Heat shock proteins/chaperonins. J Clin Rheumatol. 2002; 8:174–80.34. Li PL, Chao YM, Chan SH, Chan JY. Potentiation of baroreceptor reflex response by heat shock protein 70 in nucleus tractus solitarii confers cardiovascular protection during heatstroke. Circulation. 2001; 103:2114–9.
Article35. Yang RC, Yang SL, Chen SW, Lai SL, Chen SS, Chiang CS. Previous heat shock treatment attenuates bicuculline-induced convulsions in rats. Exp Brain Res. 1996; 108:18–22.
Article36. Nakada J, Matsura T, Okazaki N, Nishida T, Togawa A, Minami Y, et al. Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and heat shock protein 70 induction. Shock. 2005; 24:482–7.
Article37. Masuda Y, Sumita S, Fujimura N, Namiki A. Geranylgeranylacetone attenuates septic diaphragm dysfunction by induction of heat shock protein 70. Crit Care Med. 2003; 31:2585–91.
Article38. Yang SL, Jing SH, Chen SS, Chen TJ, Yang RC. The effect of hyperthermic treatment on electroencephalographic recovery after interruption of respiration in rats. Exp Brain Res. 1994; 99:431–4.
Article39. Kiang JG. Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury. Cell Res. 2004; 14:450–9.
Article40. Sumioka I, Matsura T, Kai M, Yamada K. Potential roles of hepatic heat shock protein 25 and 70i in protection of mice against acetaminophen-induced liver injury. Life Sci. 2004; 74:2551–61.
Article41. Su F, Nguyen ND, Wang Z, Cai Y, Rogiers P, Vincent JL. Fever control in septic shock: beneficial or harmful? Shock. 2005; 23:516–20.42. Singleton KD, Serkova N, Beckey VE, Wischmeyer PE. Glutamine attenuates lung injury and improves survival after sepsis: role of enhanced heat shock protein expression. Crit Care Med. 2005; 33:1206–13.
Article43. Sanli A, Onen A, Sarioglu S, Sis B, Guneli E, Gokcen B, et al. Glutamine administration enhances the healing of lung parenchymal injuries and reduces air leakage in rats. Tohoku J Exp Med. 2006; 210:239–45.
Article44. Doruk N, Buyukakilli B, Atici S, Cinel I, Cinel L, Tamer L, et al. Oral The effect of preventive use of alanyl-glutamine on diaphragm muscle function in cecal ligation and puncture-induced sepsis model. JPEN J Parenter Enteral Nutr. 2005; 29:36–43.45. Kim DJ, Park SH, Sheen MR, Jeon US, Kim SW, Koh ES, et al. Comparison of experimental lung injury from acute renal failure with injury due to sepsis. Respiration. 2006; 73:815–24.
Article46. Torres-Duen ̃as D, Benjamim CF, Ferreira SH, Cunha FQ. Failure of neutrophil migration to infectious focus and cardiovascular changes on sepsis in rats: effects of the inhibition of nitric oxides production, removal of infectious focus, and antimicrobial treatment. Shock. 2006; 25:267–76.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Heat Shock Protein 70 on Inducible Nitric Oxide Synthase during Sepsis in Rats
- The Role of Inducible 70-kDa Heat Shock Protein in Cell Cycle Control, Differentiation, and Apoptotic Cell Death of the Human Myeloid Leukemic HL-60 Cells
- Teat Shock Response Ingibits IFN-gamma Plus LPS - Induced NO Synthase Expression in Murine Peritoneal Macrophages
- Involvement of Fibronectin in the Migration of Macrophage and Expression of Nitric Oxide Synthase in the BCG induced Inflammatory Sites in Rat Bladder
- Correlation of Nitric Oxide and Corticosteroids Along the Course of Sepsis