Tuberc Respir Dis.
2009 Aug;67(2):95-104.
The Effects of Nuclear Factor-kappa B Decoy Oligodeoxynucleotide on Lipopolysaccharide-Induced Direct Acute Lung Injury
- Affiliations
-
- 1Division of Pulmonary, Sleep and Critical Care Medicine, Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea.
- 2Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Seoul, Korea. kkhchest@korea.ac.kr
- 3Division of Respiratory and Critical Care Medicine, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea.
Abstract
- BACKGROUND
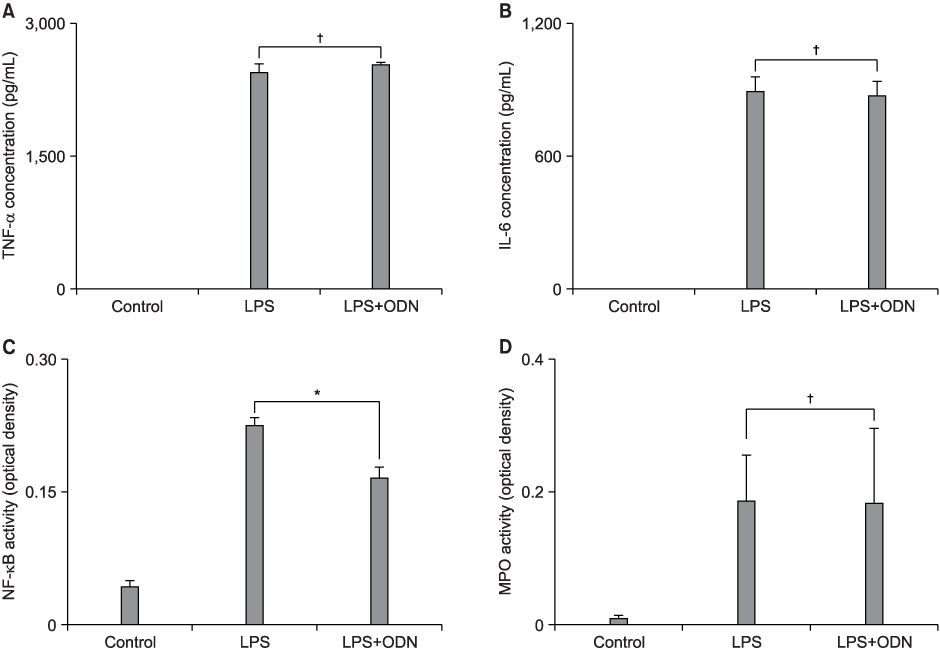
The pathophysiologic mechanisms of early acute lung injury (ALI) differ according to the type of primary insult. It is important to differentiate between direct and indirect pathophysiologic pathways, and this may influence the approach to treatment strategies. NF-kappa B decoy oligodeoxynucleotide (ODN) is a useful tool for the blockade of the expression of NF-kappa B-dependent proinflammatory mediators and has been reported to be effective in indirect ALI. The purpose of this study was to investigate the effect of NF-kappa B decoy ODN in the lipopolysaccharide (LPS)-induced direct ALI model. METHODS: Five-week-old specific pathogen-free male BALB/c mice were used for the experiment. In the preliminary studies, tumor necrosis factor (TNF)-alpha, interleukine (IL)-6 and NF-kappa B activity peaked at 6 hours after LPS administration. Myeloperoxidase (MPO) activity and ALI score were highest at 36 and 48 hours, respectively. Therefore, it was decided to measure each parameter at the time of its highest level. The study mice were randomly divided into three experimental groups: (1) control group which was administered 50 microliter of saline and treated with intratracheal administration of 200 microliter DW containing only hemagglutinating virus of Japan (HVJ) vector (n=24); (2) LPS group in which LPS-induced ALI mice were treated with intratracheal administration of 200 microliter DW containing only HVJ vector (n=24); (3) LPS+ODN group in which LPS-induced ALI mice were treated with intratracheal administration of 200 microliter DW containing 160 microgram of NF-kappa B decoy ODN and HVJ vector (n=24). Each group was subdivided into four experimental subgroups: (1) tissue subgroup for histopathological examination for ALI at 48 hours (n=6); (2) 6-hour bronchoalveolar lavage (BAL) subgroup for measurement of TNF-alpha and IL-6 in BAL fluid (BALF) (n=6); (3) 36-hour BAL subgroup for MPO activity assays in BALF (n=6); and (4) tissue homogenate subgroup for measurement of NF-kappa B activity in lung tissue homogenates at 6 hours (n=6). RESULTS: NF-kappa B decoy ODN treatment significantly decreased NF-kappa B activity in lung tissues. However, it failed to improve the parameters of LPS-induced direct ALI, including the concentrations of tumor necrosis factor-alpha and interleukin-6 in BALF, myeloperoxidase activity in BALF and histopathologic changes measured by the ALI score. CONCLUSION: NF-kappa B decoy ODN, which has been proven to be effective in indirect models, had no effect in the direct ALI model.
Keyword
MeSH Terms
-
Acute Lung Injury
Animals
Bronchoalveolar Lavage
Humans
Inflammation
Interleukin-6
Interleukins
Lipopolysaccharides
Lung
Male
Mice
NF-kappa B
Oligodeoxyribonucleotides
Peroxidase
Sendai virus
Tumor Necrosis Factor-alpha
Interleukin-6
Interleukins
Lipopolysaccharides
NF-kappa B
Oligodeoxyribonucleotides
Peroxidase
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994. 149:818–824.2. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005. 353:1685–1693.3. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000. 342:1301–1308.4. Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001. 164:1701–1711.5. Leeper-Woodford SK, Detmer K. Acute hypoxia increases alveolar macrophage tumor necrosis factor activity and alters NF-kappaB expression. Am J Physiol. 1999. 276:L909–L916.6. Moine P, McIntyre R, Schwartz MD, Kaneko D, Shenkar R, Le Tulzo Y, et al. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock. 2000. 13:85–91.7. Pepperl S, Dörger M, Ringel F, Kupatt C, Krombach F. Hyperoxia upregulates the NO pathway in alveolar macrophages in vitro: role of AP-1 and NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2001. 280:L905–L913.8. Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, et al. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol. 1998. 275:L1040–L1050.9. Shenkar R, Yum HK, Arcaroli J, Kupfner J, Abraham E. Interactions between CBP, NF-kappaB, and CREB in the lungs after hemorrhage and endotoxemia. Am J Physiol Lung Cell Mol Physiol. 2001. 281:L418–L426.10. Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999. 274:27339–27342.11. Nathens AB, Bitar R, Davreux C, Bujard M, Marshall JC, Dackiw AP, et al. Pyrrolidine dithiocarbamate attenuates endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol. 1997. 17:608–616.12. Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, et al. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997. 272:21096–21103.13. Sheehan M, Wong HR, Hake PW, Malhotra V, O'Connor M, Zingarelli B. Parthenolide, an inhibitor of the nuclear factor-kappaB pathway, ameliorates cardiovascular derangement and outcome in endotoxic shock in rodents. Mol Pharmacol. 2002. 61:953–963.14. Bielinska A, Shivdasani RA, Zhang LQ, Nabel GJ. Regulation of gene expression with double-stranded phosphorothioate oligonucleotides. Science. 1990. 250:997–1000.15. Matsuda N, Hattori Y, Jesmin S, Gando S. Nuclear factor-kappaB decoy oligodeoxynucleotides prevent acute lung injury in mice with cecal ligation and puncture-induced sepsis. Mol Pharmacol. 2005. 67:1018–1025.16. Morishita R, Higaki J, Tomita N, Ogihara T. Application of transcription factor "decoy" strategy as means of gene therapy and study of gene expression in cardiovascular disease. Circ Res. 1998. 82:1023–1028.17. Igwe OJ. Modulation of peripheral inflammation in sensory ganglia by nuclear factor (kappa)B decoy oligodeoxynucleotide: involvement of SRC kinase pathway. Neurosci Lett. 2005. 381:114–119.18. Nakashima H, Aoki M, Miyake T, Kawasaki T, Iwai M, Jo N, et al. Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligodeoxynucleotides suppressing activity of nuclear factor kappaB and ets transcription factors. Circulation. 2004. 109:132–138.19. Ogushi I, Iimuro Y, Seki E, Son G, Hirano T, Hada T, et al. Nuclear factor kappa B decoy oligodeoxynucleotides prevent endotoxin-induced fatal liver failure in a murine model. Hepatology. 2003. 38:335–344.20. Xu MQ, Shuai XR, Yan ML, Zhang MM, Yan LN. Nuclear factor-kappaB decoy oligodeoxynucleotides attenuates ischemia/reperfusion injury in rat liver graft. World J Gastroenterol. 2005. 11:6960–6967.21. Yokoseki O, Suzuki J, Kitabayashi H, Watanabe N, Wada Y, Aoki M, et al. cis Element decoy against nuclear factor-kappaB attenuates development of experimental autoimmune myocarditis in rats. Circ Res. 2001. 89:899–906.22. Matsuda N, Hattori Y, Takahashi Y, Nishihira J, Jesmin S, Kobayashi M, et al. Therapeutic effect of in vivo transfection of transcription factor decoy to NF-kappaB on septic lung in mice. Am J Physiol Lung Cell Mol Physiol. 2004. 287:L1248–L1255.23. Pelosi P, D'Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003. 42:48s–56s.24. Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977. 116:589–615.25. Blaisdell FW. Pathophysiology of the respiratory distress syndrome. Arch Surg. 1974. 108:44–49.26. Lamy ML, Fallat RJ, Koeniger EL, Dietrich HP, Kamm B, Hill JD. Pathophysiology of adult respiratory distress syndrome. Acta Anaesthesiol Belg. 1975. 23:Suppl. 64–77.27. Nash G, Foley FD, Langlinais PC. Pulmonary interstitial edema and hyaline membranes in adult burn patients. Electron microscopic observations. Hum Pathol. 1974. 5:149–160.28. Callister ME, Evans TW. Pulmonary versus extrapulmonary acute respiratory distress syndrome: different diseases or just a useful concept? Curr Opin Crit Care. 2002. 8:21–25.29. Rocco PR, Zin WA. Pulmonary and extrapulmonary acute respiratory distress syndrome: are they different? Curr Opin Crit Care. 2005. 11:10–17.30. Kim JH, Suk MH, Yoon DW, Lee SH, Hur GY, Jung KH, et al. Inhibition of matrix metalloproteinase-9 prevents neutrophilic inflammation in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2006. 291:L580–L587.31. Hirano S. Migratory responses of PMN after intraperitoneal and intratracheal administration of lipopolysaccharide. Am J Physiol. 1996. 270:L836–L845.32. Harada K, Ohira S, Isse K, Ozaki S, Zen Y, Sato Y, et al. Lipopolysaccharide activates nuclear factor-kappaB through toll-like receptors and related molecules in cultured biliary epithelial cells. Lab Invest. 2003. 83:1657–1667.33. Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, et al. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001. 29:E21.34. Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element "decoy" against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997. 3:894–899.35. Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N, et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther. 2002. 6:219–226.36. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.37. Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005. 98:1777–1783.38. Yoshimura S, Morishita R, Hayashi K, Yamamoto K, Nakagami H, Kaneda Y, et al. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element 'decoy' of nuclear factor-kappaB binding site as a novel molecular strategy. Gene Ther. 2001. 8:1635–1642.39. Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006. 12:151–170.40. Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, et al. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003. 167:1027–1035.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intra-peritoneal NF-kappaB decoy oligodeoxynucleotide decreases postoperative intra-abdominal adhesion
- The Effect of Ring-type NF-kappa B(NF-kB) Decoy Oligodeoxynucleotide on the Kidney for an Experimental Unilateral Ureteral Obstruction in Mice
- Artemisia argyi attenuates airway inflammation in lipopolysaccharide induced acute lung injury model
- Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) Augments Acute Lung Injury via Its Neutrophil Priming Effects
- The Role of Transglutaminase-2 in Fibroproliferation after Lipopolysaccharide-induced Acute Lung Injury