Lab Anim Res.
2017 Sep;33(3):209-215. 10.5625/lar.2017.33.3.209.
Artemisia argyi attenuates airway inflammation in lipopolysaccharide induced acute lung injury model
- Affiliations
-
- 1College of Veterinary Medicine (BK21 project team), Chonnam National University, Gwangju, Korea. dvmmk79@gmail.com
- 2Natural Medicine Research Center, Korea Research Institute of Bioscience & Biotechnology, Chungju, Korea.
- 3Namhae Garlic Research Institute, Namhae-gun, Korea.
- 4Korea Ginseng Corporation, Daejeon, Korea. hsleei@naver.com
- KMID: 2391093
- DOI: http://doi.org/10.5625/lar.2017.33.3.209
Abstract
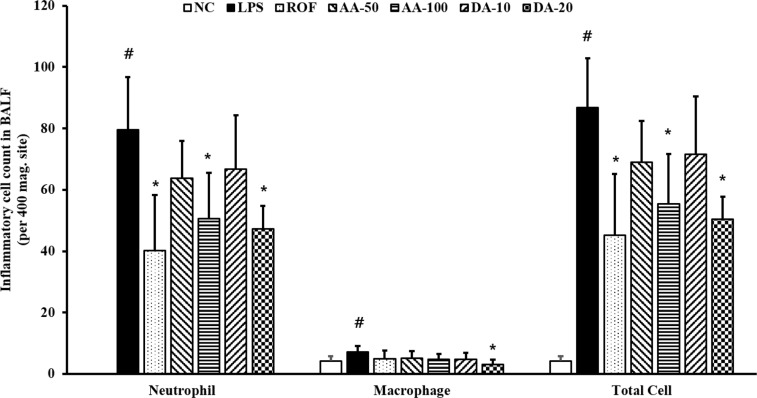
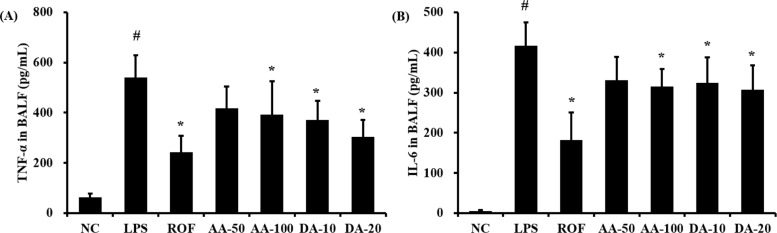
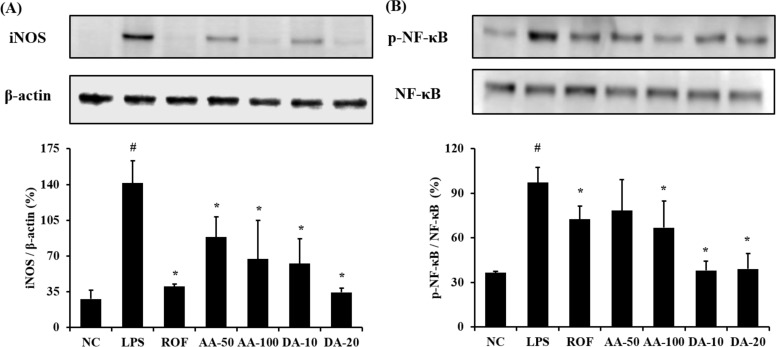
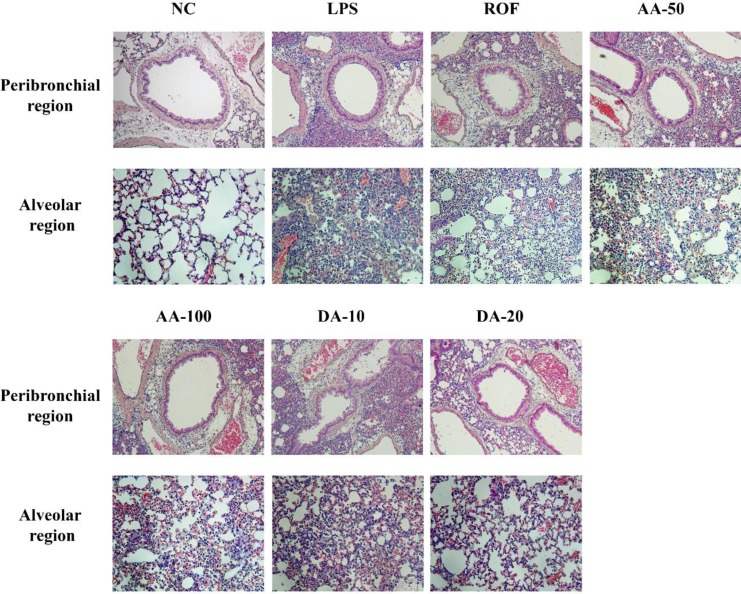
- Artemisia argyi is used as a health supplement, tea, and food source in Korea. This study aimed to evaluate the effect of Artemisia argyi (AA) and its active compound, dehydromatricarin A (DA), on the attenuation of airway inflammation in a murine model of lipopolysaccharide (LPS)-induced acute lung injury (ALI). The C57BL/6 mice were administered AA (50 mg/kg or 100 mg/kg) and DA (10 mg/kg or 20 mg/kg) by oral gavage from day 0 to 7 days and LPS treated by intranasal instillation 48 hours before the sacrifice. The treatment of AA and DA markedly decreased inflammatory cells in the bronchoalveolar lavage fluid (BALF) compared with that in ALI-induced mice, which was accompanied by a significant reduction in the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in BALF. Furthermore, the administration of AA and DA clearly decreased inducible nitric oxide synthase (iNOS) expression and nuclear factor kappa B (NF-κB) phosphorylation in comparison with that in the ALI-induced mice. The histological examination of the lung tissue revealed that the administration of AA and DA suppressed the inflammatory cell infiltration into the peribronchial and alveolar lesions induced by LPS instillation. Collectively, our results indicated that AA and DA effectively decreased the airway inflammatory response induced by LPS instillation. Therefore, AA and DA may offer a potential therapy for airway inflammatory disease.
Keyword
MeSH Terms
Figure
Reference
-
1. Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007; 4(9):e269. PMID: 17803352.
Article2. Xiao Q, Dong N, Yao X, Wu D, Lu Y, Mao F, Zhu J, Li J, Huang J, Chen A, Huang L, Wang X, Yang G, He G, Xu Y, Lu W. Bufexamac ameliorates LPS-induced acute lung injury in mice by targeting LTA4H. Sci Rep. 2016; 6:25298. PMID: 27126280.
Article3. Yang SC, Chang SH, Hsieh PW, Huang YT, Ho CM, Tsai YF, Hwang TL. Dipeptide HCH6-1 inhibits neutrophil activation and protects against acute lung injury by blocking FPR1. Free Radic Biol Med. 2017; 106:254–269. PMID: 28232203.
Article4. Pedrazza L, Cunha AA, Luft C, Nunes NK, Schimitz F, Gassen RB, Breda RV, Donadio MV, de Souza Wyse AT, Pitrez PMC, Rosa JL, de Oliveira JR. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol. 2017; 232(12):3552–3564. PMID: 28112391.
Article5. Yingkun N, Zhenyu W, Jing L, Xiuyun L, Huimin Y. Stevioside protects LPS-induced acute lung injury in mice. Inflammation. 2013; 36(1):242–250. PMID: 22968433.
Article6. Al-Harbi NO, Imam F, Al-Harbi MM, Ansari MA, Zoheir KM, Korashy HM, Sayed-Ahmed MM, Attia SM, Shabanah OA, Ahmad SF. Dexamethasone Attenuates LPS-induced Acute Lung Injury through Inhibition of NF-κB, COX-2, and Pro-inflammatory Mediators. Immunol Invest. 2016; 45(4):349–369. PMID: 27104958.
Article7. Wang T, Hou W, Fu Z. Preventative effect of OMZ-SPT on lipopolysaccharide-induced acute lung injury and inflammation via nuclear factor-kappa B signaling in mice. Biochem Biophys Res Commun. 2017; 485(2):284–289. PMID: 28223218.
Article8. Wu Y, Jin F, Wang Y, Li F, Wang L, Wang Q, Ren Z, Wang Y. In vitro and in vivo anti-inflammatory effects of theaflavin-3,3'-digallate on lipopolysaccharide-induced inflammation. Eur J Pharmacol. 2017; 794:52–60. PMID: 27871911.
Article9. Zhang B, Liu ZY, Li YY, Luo Y, Liu ML, Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG, Li ZC. Antiinflammatory effects of matrine in LPS-induced acute lung injury in mice. Eur J Pharm Sci. 2011; 44(5):573–579. PMID: 22019524.
Article10. Yang S, Yu Z, Wang L, Yuan T, Wang X, Zhang X, Wang J, Lv Y, Du G. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J Ethnopharmacol. 2017; 200:147–155. PMID: 28192201.
Article11. Shen B, Zhao C, Chen C, Li Z, Li Y, Tian Y, Feng H. Picroside II Protects Rat Lung and A549 Cell Against LPS-Induced Inflammation by the NF-κB Pathway. Inflammation. 2017; 40(3):752–761. PMID: 28161732.
Article12. Li N, Song Y, Zhao W, Han T, Lin S, Ramirez O, Liang L. Small interfering RNA targeting NF-κB attenuates lipopolysaccharideinduced acute lung injury in rats. BMC Physiol. 2016; 16(1):7. PMID: 28031043.
Article13. Shin NR, Shin IS, Song HH, Hong JM, Kwon OK, Jeon CM, Kim JH, Lee SW, Lee JK, Jin H, Li WY, Oh SR, Hahn KW, Ahn KS. Callicarpa japonica Thunb. reduces inflammatory responses: a mouse model of lipopolysaccharide-induced acute lung injury. Int Immunopharmacol. 2015; 26(1):174–180. PMID: 25662753.
Article14. Kim JK, Shin EC, Lim HJ, Choi SJ, Kim CR, Suh SH, Kim CJ, Park GG, Park CS, Kim HK, Choi JH, Song SW, Shin DH. Characterization of Nutritional Composition, Antioxidative Capacity, and Sensory Attributes of Seomae Mugwort, a Native Korean Variety of Artemisia argyi H. Lév. & Vaniot. J Anal Methods Chem. 2015; 2015:916346. PMID: 26550520.15. Yun C, Jung Y, Chun W, Yang B, Ryu J, Lim C, Kim JH, Kim H, Cho SI. Anti-Inflammatory Effects of Artemisia Leaf Extract in Mice with Contact Dermatitis In Vitro and In Vivo. Mediators Inflamm. 2016; 2016:8027537. PMID: 27647952.16. Lee NY, Chung KS, Jin JS, Bang KS, Eom YJ, Hong CH, Nugroho A, Park HJ, An HJ. Effect of Chicoric Acid on Mast Cell-Mediated Allergic Inflammation in Vitro and in Vivo. J Nat Prod. 2015; 78(12):2956–2962. PMID: 26593037.
Article17. Yao X, Wu D, Dong N, Ouyang P, Pu J, Hu Q, Wang J, Lu W, Huang J, Moracin C. A Phenolic Compound Isolated from Artocarpus heterophyllus, Suppresses Lipopolysaccharide-Activated Inflammatory Responses in Murine Raw264.7 Macrophages. Int J Mol Sci. 2016; 17(8):18. Shin NR, Ryu HW, Ko JW, Park SH, Yuk HJ, Kim HJ, Kim JC, Jeong SH, Shin IS. Artemisia argyi attenuates airway inflammation in ovalbumin-induced asthmatic animals. J Ethnopharmacol. 2017; 209:108–115. PMID: 28735728.
Article19. Seehase S, Lauenstein HD, Schlumbohm C, Switalla S, Neuhaus V, Förster C, Fieguth HG, Pfennig O, Fuchs E, Kaup FJ, Bleyer M, Hohlfeld JM, Braun A, Sewald K, Knauf S. LPS-induced lung inflammation in marmoset monkeys-an acute model for antiinflammatory drug testing. PLoS One. 2012; 7(8):e43709. PMID: 22952743.20. Yuan X, Wang Y, Du D, Hu Z, Xu M, Xu M, Liu Z. The effects of the combination of sodium ferulate and oxymatrine on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2012; 35(3):1161–1168. PMID: 22219049.
Article21. Kang P, Kim KY, Lee HS, Min SS, Seol GH. Anti-inflammatory effects of anethole in lipopolysaccharide-induced acute lung injury in mice. Life Sci. 2013; 93(24):955–961. PMID: 24404587.
Article22. Han HJ, Li M, Son JK, Seo CS, Song SW, Kwak SH, Bae HB. Sauchinone, a lignan from Saururus chinensis, attenuates neutrophil pro-inflammatory activity and acute lung injury. Int Immunopharmacol. 2013; 17(2):471–477. PMID: 23928505.
Article23. Niu X, Hu H, Li W, Li Y, Huang H, Mu Q, Yao H, Li H. Protective effect of total alkaloids on lipopolysaccharide-induced acute lung injury. J Surg Res. 2014; 189(1):126–134. PMID: 24594217.
Article24. Park HJ, Jeon BT, Kim HC, Roh GS, Shin JH, Sung NJ, Han J, Kang D. Aged red garlic extract reduces lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages and acute pulmonary inflammation through haeme oxygenase-1 induction. Acta Physiol (Oxf). 2012; 205(1):61–70. PMID: 22353229.
Article25. Sun Q, Chen L, Gao M, Jiang W, Shao F, Li J, Wang J, Kou J, Yu B. Ruscogenin inhibits lipopolysaccharide-induced acute lung injury in mice: involvement of tissue factor, inducible NO synthase and nuclear factor (NF)-κB. Int Immunopharmacol. 2012; 12(1):88–93. PMID: 22079591.
Article26. Zhang X, Huang H, Yang T, Ye Y, Shan J, Yin Z, Luo L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury. 2010; 41(7):746–752. PMID: 20227691.
Article27. Wu Q, Sun G, Yuan X, Soromou LW, Chen N, Xiong Y, Feng H. Tubeimoside-1 attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Immunopharmacol Immunotoxicol. 2013; 35(4):514–523. PMID: 23844578.
Article28. Gao M, Chen L, Yu H, Sun Q, Kou J, Yu B. Diosgenin down-regulates NF-κB p65/p50 and p38MAPK pathways and attenuates acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol. 2013; 15(2):240–245. PMID: 23246979.
Article29. Chi G, Wei M, Xie X, Soromou LW, Liu F, Zhao S. Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation. 2013; 36(2):501–511. PMID: 23180366.
Article30. Chen L, Zhao L, Zhang C, Lan Z. Protective effect of p-cymene on lipopolysaccharide-induced acute lung injury in mice. Inflammation. 2014; 37(2):358–364. PMID: 24085645.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical Composition of Artemisia argyi Extract (RW0117) and Protective Effects against Gastric Lesions in vivo
- Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome
- Potential Moracin M Prodrugs Strongly Attenuate Airway Inflammation In Vivo
- Development of Analytical Method and Validation using HPLC/PDA for Discrimination between Artemisiae Argyi Folium and Artemisiae Iwayomogii Herba
- EphA2 Receptor Signaling Mediates Inflammatory Responses in Lipopolysaccharide-Induced Lung Injury