Nat Prod Sci.
2016 Mar;22(1):70-75. 10.20307/nps.2016.22.1.70.
The New Phytoformula Containing Morus alba, Schizandra sinensis and Asparagus cochinchinensis Inhibits Lung Inflammation in vitro and in vivo
- Affiliations
-
- 1College of Pharmacy, Kangwon National University, Chunchon 24341, Korea. hpkim@kangwon.ac.kr
- 2Ernest Mario School of Pharmacy Rutgers, The State University of New Jersey, USA.
- KMID: 2312925
- DOI: http://doi.org/10.20307/nps.2016.22.1.70
Abstract
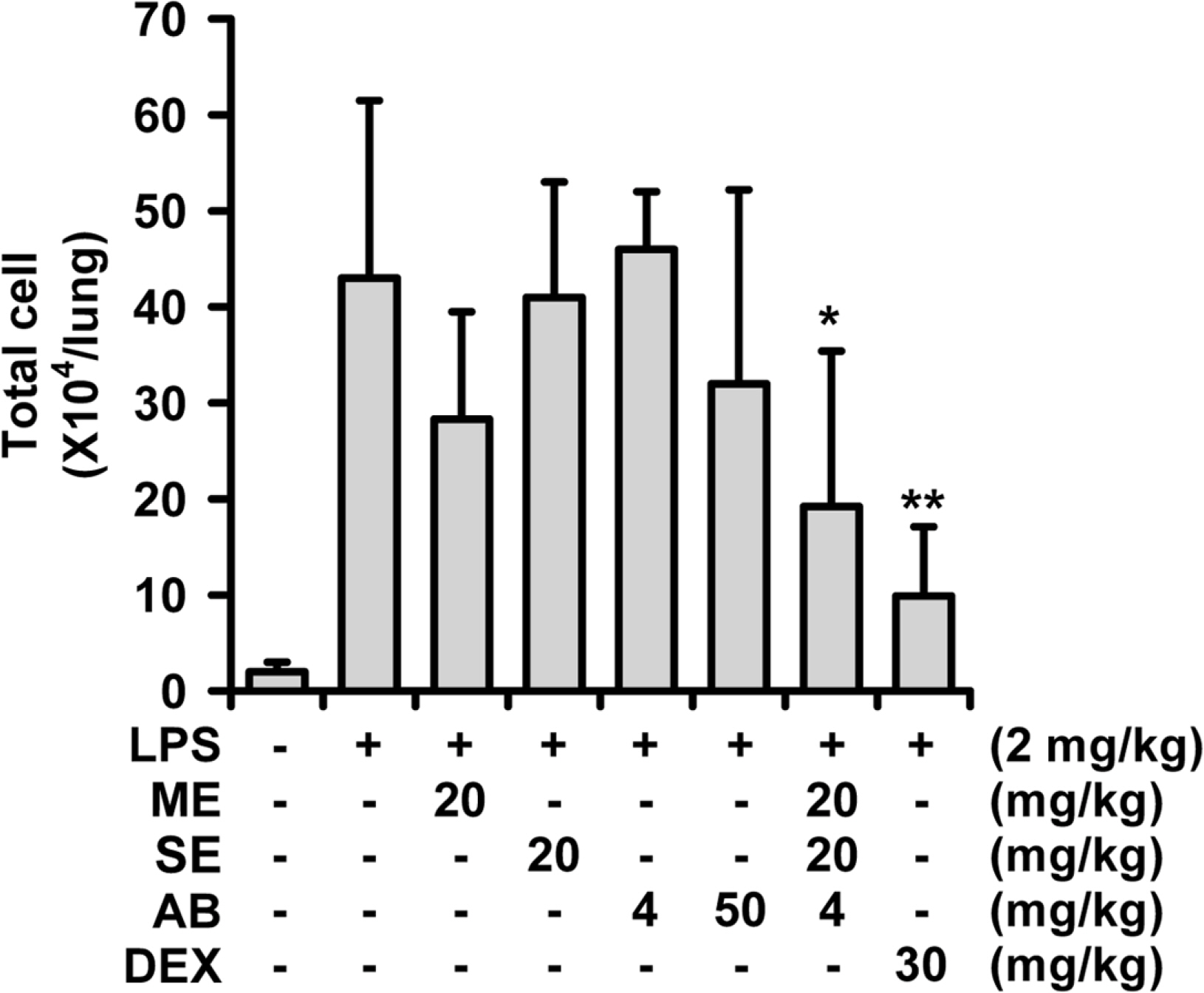
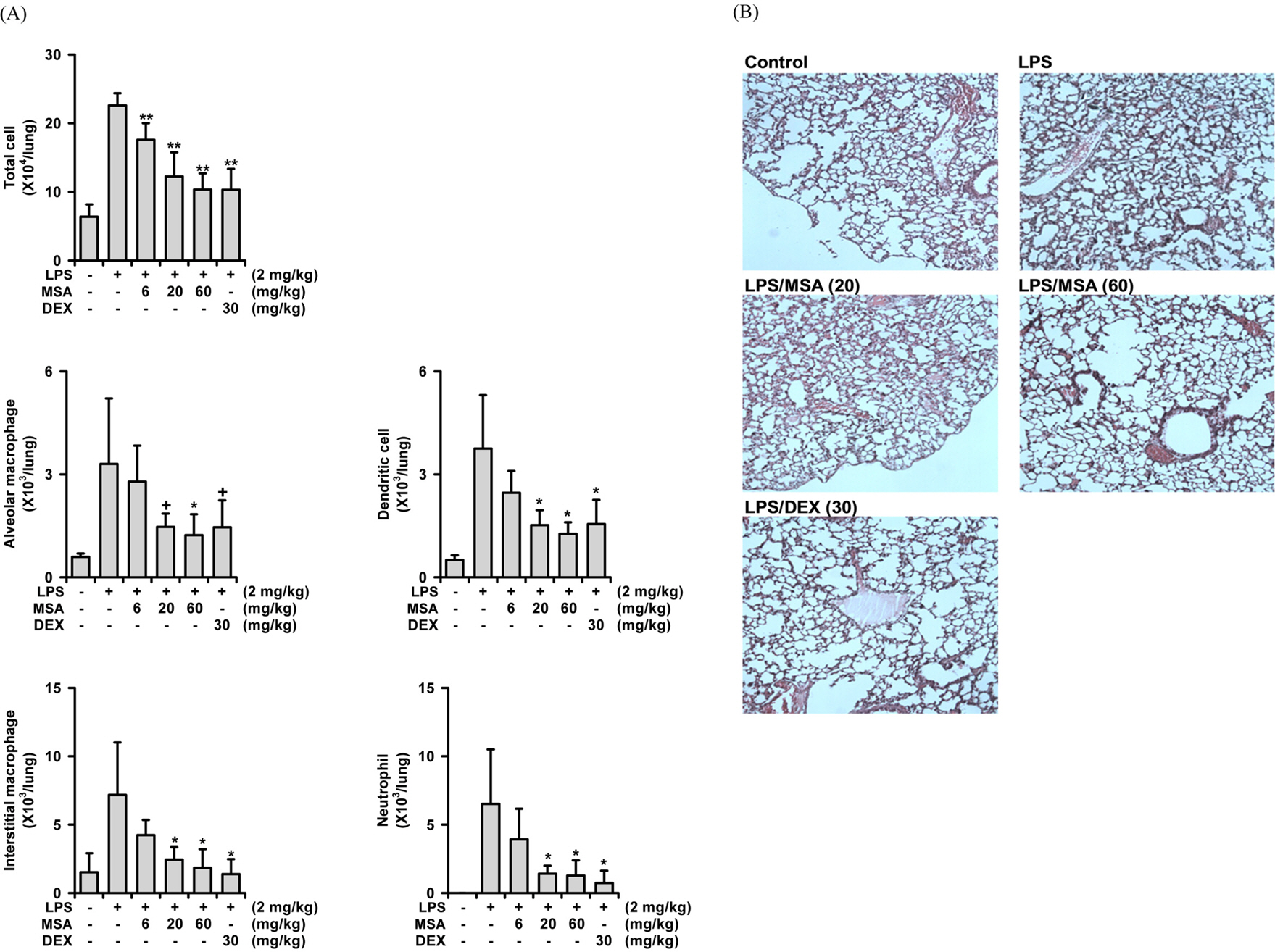
- A phytoformula containing the root barks of Morus alba, the fructus of Schizandra sinensis and the roots of Asparagus cochinchinensis (MSA) was prepared as a potential new herbal remedy, and its therapeutic potential for alleviating inflammatory lung conditions was examined. For in vivo evaluation, an animal model of lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice was used. With oral administration of 6 - 60 mg/kg, MSA potently and dose-dependently inhibited bronchitis-like symptoms in acute lung injury induced by intranasal treatment of LPS as judged by the number of cells in the bronchoalveolar lavage fluid (BALF) and histological observation. The inhibitory potency was comparable with that of dexamethasone. For in vitro assay, the effects on the production of proinflammatory molecules in lung epithelial cells and alveolar macrophages were examined. Although MSA inhibited IL-6 production in IL-1β-treated lung epithelial cells (A549) only at a high concentration (300 µg/ml), the formula strongly and concentration-dependently inhibited NO production in LPS-treated alveolar macrophages (MH-S) at 20 - 300 µg/ml. Based on all of these findings, the new phytoformula MSA is suggested to have the potential to control inflammatory lung diseases including bronchitis, at least in part, by inhibiting inducible nitric oxide synthase-catalyzed NO production.
MeSH Terms
Figure
Reference
-
(1). Jeffery P. K. Am. J.Respir. Crit. Care Med. 2001; 164:S28–S38.(2). Barnes P. J.Clin. Chest Med. 2014; 35:71–86.(3). Bae K.The medicinal plants of Korea. Kyo-Hak Pub. Co.;Korea: 2000. p. 73.(4). Hong C. H., Hur S. K., Oh O. J., Kim S. S., Nam K. A, Lee S. K. J.Ethnopharmacol. 2002; 83:153–159.(5). Chao W. W., Kuo Y. H., Li W. C., Lin B. F. J.Ethnopharmacol. 2009; 122:68–75.(6). Cheon B. S., Kim Y. H., Son K. S., Chang H. W., Kang S. S., Kim H. P.Planta Med. 2000; 66:596–600.(7). Yang Z. G., Matsuzaki K., Takamatsu S., Kitanaka S.Molecules. 2011; 16:6010–6022.(8). Lim H. J., Jin H. G., Woo E. R., Lee S. K., Kim H. P. J.Ethnopharmacol. 2013; 149:169–175.(9). Bae K.The medicinal plants of Korea. Kyo-Hak Pub. Co.;Korea: 2000. p. 116.(10). Lim H., Son K. H., Bae K. H., Hung T. M., Kim Y. S., Kim H. P.Phytother. Res. 2009; 23:1489–1492.(11). Kim H., Ahn Y. T., Kim Y. S., Cho S. I., An W. G.Pharmacogn. Mag. 2014; 10:S80–S85.(12). Bae H., Kim R., Kim Y., Lee E., Kim H. J., Jang Y. P., Jung S. K., Kim J. J.Ethnopharmacol. 2012; 142:41–47.(13). Lee H. J., Park J. S., Yoon Y. P., Shin Y. J., Lee S. K., Kim Y. S., Hong J. H., Son K. H., Lee C. J.Phytomedicine. 2015; 22:568–572.(14). Lee J. H., Lim H. J., Lee C. W., Son K. H., Son J. K., Lee S. K., Kim H. P.Evid. Based Complement Alternat. Med. 2015; DOI: doi:10.1155/2015/640846.(15). Mosmann T. J.Immunol. Methods. 1983; 65:55–63.(16). Ko H. J., Jin J. H., Kwon O. S., Kim J. T., Son K. H., Kim H. P.Biomol. Ther. 2011; 19:324–330.(17). Nomura T.Yakugaku Zasshi. 2001; 121:535–556.(18). Zhu L., Li B., Liu X., Huang G., Meng X.Food Chem. 2015; 186:146–152.(19). Kim S. Y., Son K. H., Chang H. W., Kang S. S., Kim H. P.Arch. Pharm. Res. 1999; 22:313–316.(20). Guo R., Pittler M. H., Ernst E.Eur. Respir. J. 2006; 28:330–338.(21). Sharma M., Arnason J. T., Burt A., Hudson J. B.Phytother. Res. 2006; 20:147–152.(22). Agbabiaka T. B., Guo R., Ernst E.Phytomedicine. 2008; 15:378–385.(23). Matthys H., Funk P.Planta Med. 2008; 74:686–692.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Morus alba L. and Natural Products Including Morusin on In Vivo Secretion and In Vitro Production of Airway MUC5AC Mucin
- Growth Inhibitory Effects of Various Herbal Extracts on Metronidazole Resistant Strain of Trichomonas vaginalis
- Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation
- Phytochemical Constituents of the Root Bark from Morus alba and Their Il-6 Inhibitory Activity
- Dose dependence and durability of the therapeutic effects of Asparagus cochinchinensis fermented extract in an ovalbumin-challenged asthma model