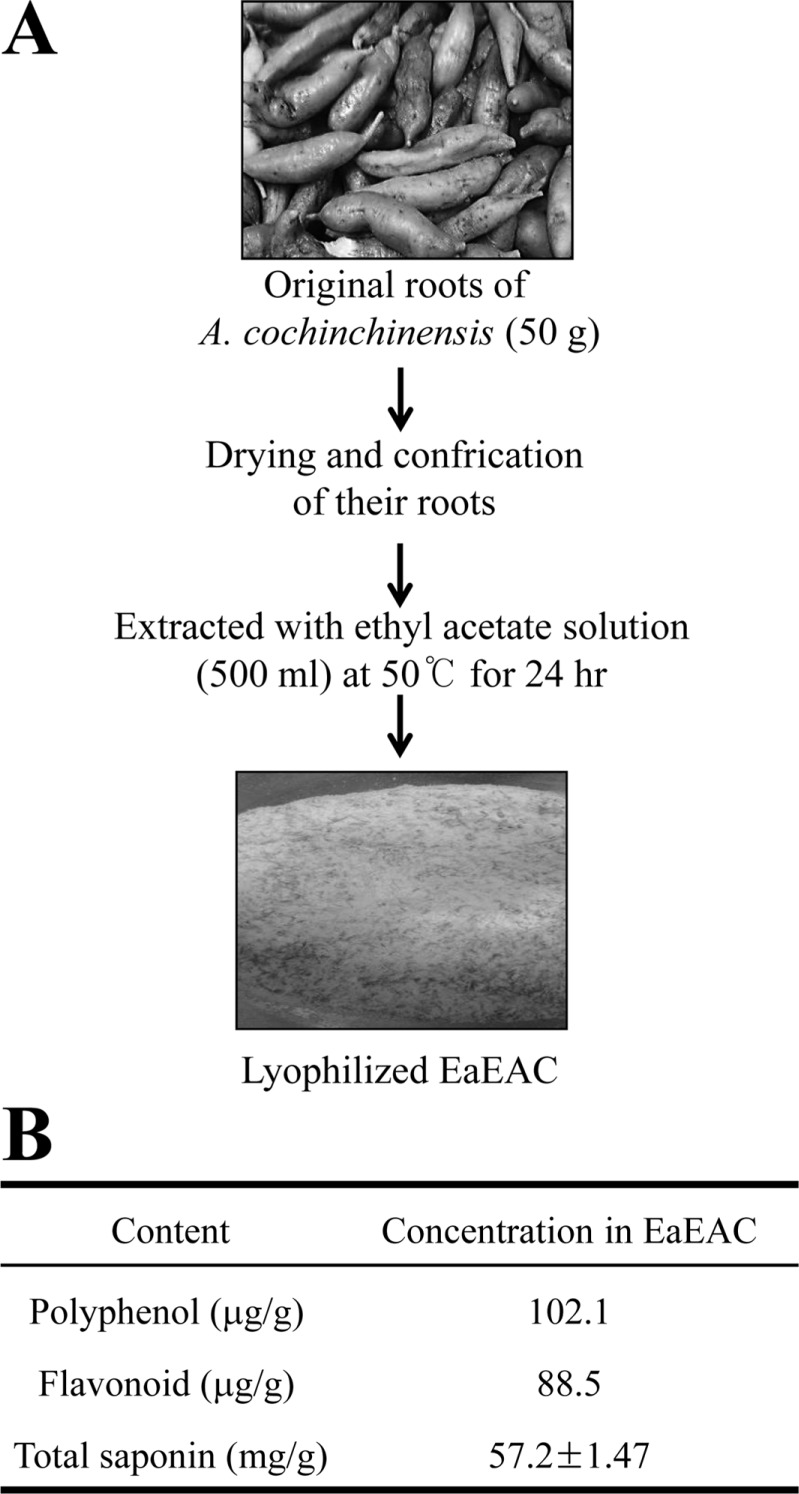
Lab Anim Res.
2016 Mar;32(1):34-45. 10.5625/lar.2016.32.1.34.
Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation
- Affiliations
-
- 1College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang 627-706, Korea. dyhwang@pusan.ac.kr
- 2Gangrim Organics, Miryang 627-706, Korea.
- 3College of Human Ecology, Pusan National University, Busan, Korea.
- KMID: 2312152
- DOI: http://doi.org/10.5625/lar.2016.32.1.34
Abstract
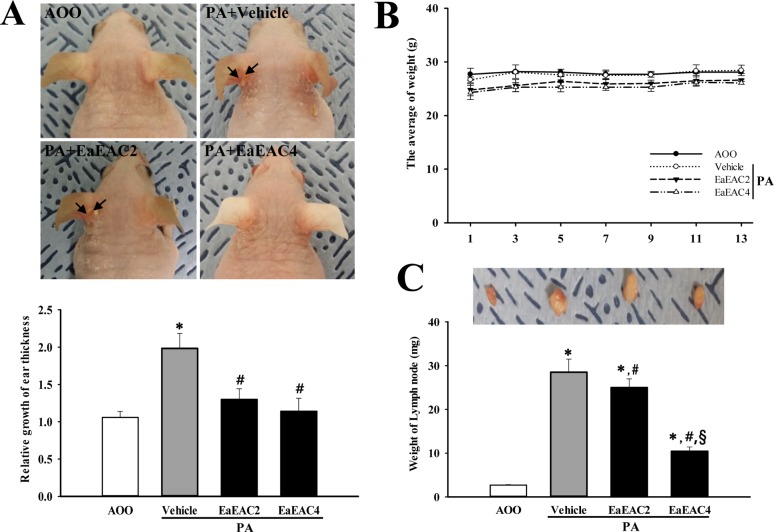
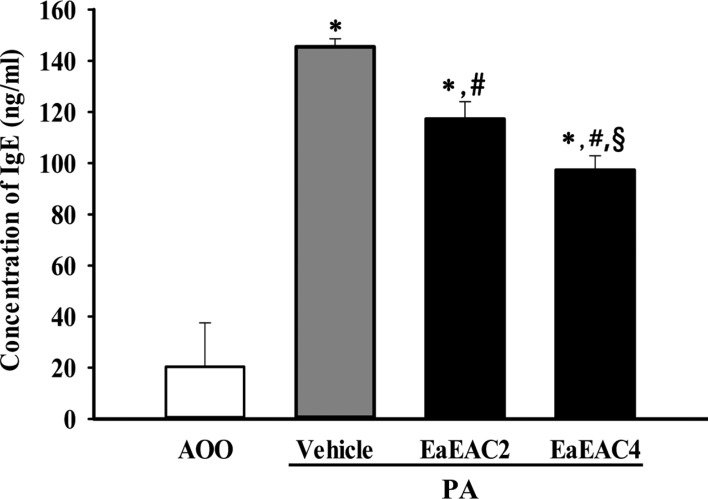
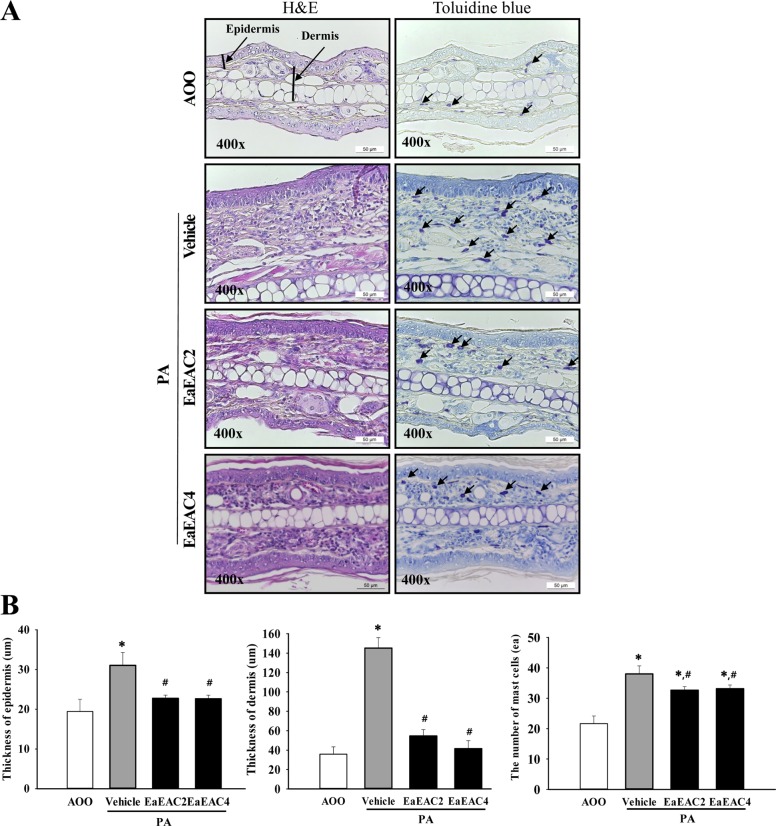
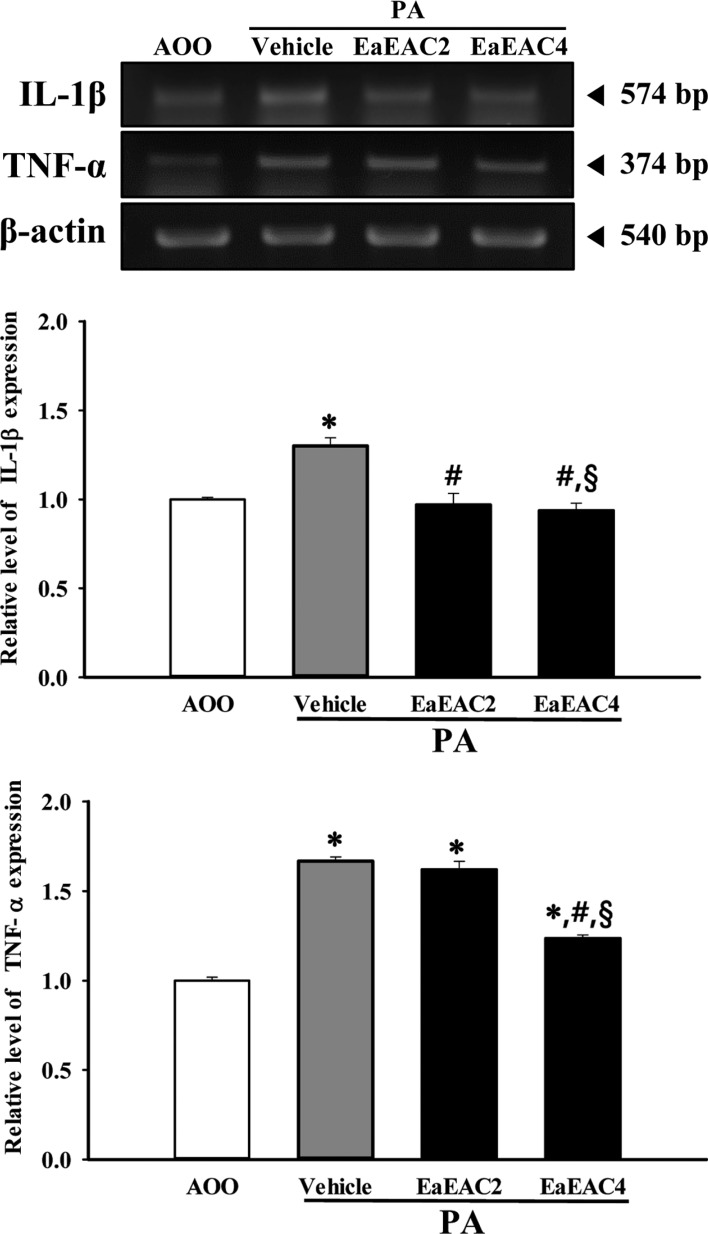
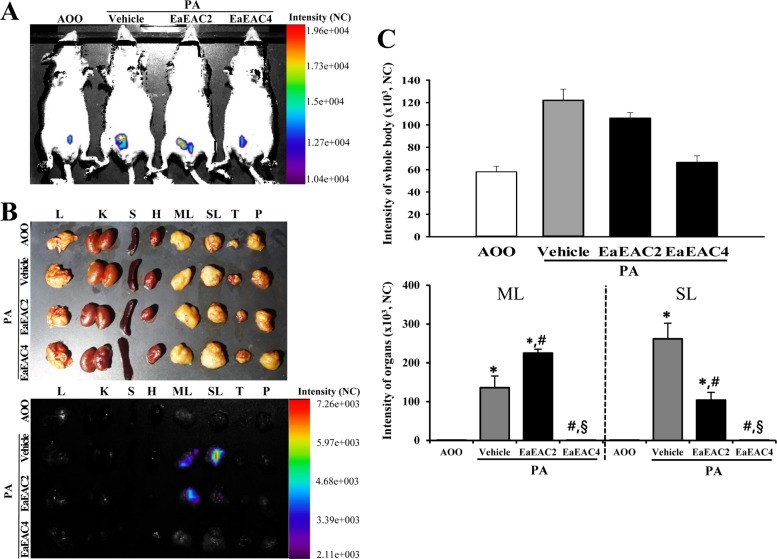
- Asparagus cochinchinensis has been used to treat various diseases including fever, cough, kidney disease, breast cancer, inflammatory disease and brain disease, while IL-4 cytokine has been considered as key regulator on the skin homeostasis and the predisposition toward allergic skin inflammation. However, few studies have investigated its effects and IL-4 correlation on skin inflammation to date. To quantitatively evaluate the suppressive effects of ethyl acetate extracts of A. cochinchinensis (EaEAC) on phthalic anhydride (PA)-induced skin inflammation and investigate the role of IL-4 during their action mechanism, alterations in general phenotype biomarkers and luciferase-derived signals were measured in IL-4/Luc/CNS-1 transgenic (Tg) mice with PA-induced skin inflammation after treatment with EaEAC for 2 weeks. Key phenotype markers including lymph node weight, immunoglobulin E (IgE) concentration, epidermis thickness and number of infiltrated mast cells were significantly decreased in the PA+EaEAC treated group compared with the PA+Vehicle treated group. In addition, expression of IL-1β and TNF-α was also decreased in the PA+EaEAC cotreated group, compared to PA+Vehicle treated group. Furthermore, a significant decrease in the luciferase signal derived from IL-4 promoter was detected in the abdominal region, submandibular lymph node and mesenteric lymph node of the PA+EaEAC treated group, compared to PA+Vehicle treated group. Taken together, these results suggest that EaEAC treatment could successfully improve PA-induced skin inflammation of IL-4/Luc/CNS-1 Tg mice, and that IL-4 cytokine plays a key role in the therapeutic process of EaEAC.
Keyword
MeSH Terms
Figure
Reference
-
1. Corsini E, Galli CL. Cytokines and irritant contact dermatitis. Toxicol Lett. 1998; 102-103:277–282. PMID: 10022266.
Article2. Guin JD. Eyelid dermatitis: experience in 203 cases. J Am Acad Dermatol. 2002; 47(5):755–765. PMID: 12399770.
Article4. Hernandez-Pando R, Rook GA. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994; 82(4):591–595. PMID: 7835922.5. Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J Antibiot (Tokyo). 1987; 40(9):1249–1255. PMID: 2445721.
Article6. Meingassner JG, Grassberger M, Fahrngruber H, Moore HD, Schuurman H, Stütz A. A novel anti-inflammatory drug, SDZ ASM 981, for the topical and oral treatment of skin diseases: in vivo pharmacology. Br J Dermatol. 1997; 137(4):568–576. PMID: 9390333.7. Hanania NA, Chapman KR, Kesten S. Adverse effects of inhaled corticosteroids. Am J Med. 1995; 98(2):196–208. PMID: 7847437.
Article8. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009; 14(2):141–153. PMID: 19594223.9. Rogerio AP, Kanashiro A, Fontanari C, da Silva EV, Lucisano-Valim YM, Soares EG, Faccioli LH. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res. 2007; 56(10):402–408. PMID: 18026696.
Article10. de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005; 49(5):405–430. PMID: 15832402.11. Rocha NF, Rios ER, Carvalho AM, Cerqueira GS, Lopes Ade A, Leal LK, Dias ML, de Sousa DP, de Sousa FC. Anti-nociceptive and anti-inflammatory activities of (-)-α-bisabolol in rodents. Naunyn Schmiedebergs Arch Pharmacol. 2011; 384(6):525–533. PMID: 21870032.
Article12. Cavet ME, Harrington KL, Vollmer TR, Ward KW, Zhang JZ. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol Vis. 2011; 17:533–542. PMID: 21364905.13. Bae MJ, Shin HS, Choi DW, Shon DH. Antiallergic effect of Trigonella foenum-graecum L. extracts on allergic skin inflammation induced by trimellitic anhydride in BALB/c mice. J Ethnopharmacol. 2012; 144(3):514–522. PMID: 23036811.14. Kwak MH, Kim JE, Hwang IS, Lee YJ, An BS, Hong JT, Lee SH, Hwang DY. Quantitative evaluation of therapeutic effect of Liriope platyphylla on phthalic anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. J Ethnopharmacol. 2013; 148(3):880–889. PMID: 23726789.15. Lee YJ, Kim JE, Kwak MH, Go J, Kim DS, Son HJ, Hwang DY. Quantitative evaluation of the therapeutic effect of fermented soybean products containing a high concentration of GABA on phthalic anhydride-induced atopic dermatitis in IL-4/Luc/CNS-1 Tg mice. Int J Mol Med. 2014; 33(5):1185–1194. PMID: 24604257.
Article16. Xiong D, Yu LX, Yan X, Guo C, Xiong Y. Effects of root and stem extracts of Asparagus cochinchinensis on biochemical indicators related to aging in the brain and liver of mice. Am J Chin Med. 2011; 39(4):719–726. PMID: 21721152.17. Li M, Fei Y, Wang JK. Studies on pharmacologic effects of Radix Asparagi. LiShiZhen Med Mater Med Res. 2005; 16:580–582.18. Qu FY, Wei XD, Li SL, Wang YM, Bai SG. Experimental study of Asparagus cochinchinensis delay aging. Acta Chin Med Pharm. 1999; 2:68–70.19. Zhao YJ, Meng XL, Li XL, Qu FY. Influence of Radix asparagi nano-pharmaceutics on NOS, NO, LPF of senile mice. Chin Wild Plant Resour. 2005; 24:49–51.20. Wen JY, Li Y, Ding SS, Li QH. Nine Pharmacological screening of medicinal plants of China Liliaceae Asparagus. J Acta Acad Med Shanghai. 1993; 20:107–111.21. Luo J, Long QD, Li CX, Li L, Huang NH. Inhibitory effects of ALWB and ACM on mice bearing tumor. J GuiYang Med Coll. 2000; 25(1):15–16.22. Koo HN, Jeong HJ, Choi JY, Choi SD, Choi TJ, Cheon YS, Kim KS, Kang BK, Park ST, Chang CH, Kim CH, Lee YM, Kim HM, An NH, Kim JJ. Inhibition of tumor necrosis factor-alpha-induced apoptosis by Asparagus cochinchinensis in Hep G2 cells. J Ethnopharmacol. 2000; 73(1-2):137–143. PMID: 11025149.23. Yu FR, Lian XZ, Guo HY. Effect of lucid asparagus extract on the regulation of blood sugar. Chin J Clin Rehabil. 2006; 10:57–59.24. Luo J, Long QD, Li CX, Li L, Huang NH, Nie M, Tang PX. Comparison of antitussive, expectorant and anti-asthmatic effect between ALWB and ACM. J GuiYang Med Coll. 1998; 23(2):132–134.25. Lv B, Liu WZ. Aspartate treatment of hemodialysis patients with hypertension in 22 cases. J Tradit Chin Med. 2004; 19:43–44.26. Xiong D, Yu LX, Yan X, Guo C, Xiong Y. Effects of root and stem extracts of Asparagus cochinchinensis on biochemical indicators related to aging in the brain and liver of mice. Am J Chin Med. 2011; 39:719–726. PMID: 21721152.27. Xiao PG. Modern Chinese material medica. Beijing: China Press;2002. p. 150.28. Liu YZ, Qu FY, Zhang PX. Effect of chloroform extract of Tiandong on the brain anti-oxidation of D-galatose-induced senile mice. Heilongjiang Med Pharm. 2001; 24:7–8.29. Ni JM, Zhao R, Wang R. Comparison on amino acid content in prepared and unprepared Asparagus cochinchinensis. Chin Tradit Herb Drugs. 1992; 23:182–183.30. Tenji K, Junzo S. Studies on the Constituents of Asparagi Radix. I. On the Structures of Furostanol Oligosides of Asparagus cochinchinensis (LOUREIO) MERRILL. Chem Pharm Bull. 1979; 27:3086–3094.31. Liang ZZ, Aquino R, De Simone F, Dini A, Schettino O, Pizza C. Oligofurostanosides from Asparagus cochinchinensis. Planta Med. 1988; 54(4):344–346. PMID: 17265283.32. Yang YC, Huang SY, Shi JG. Two new furostanol glycosides from Asparagus cochinchinensis. Chin Chem Lett. 2002; 13:1185–1188.33. Cong PZ, Su KM. Handbook of analytical chemistry. Beijing: In Chemical Industry Publishing House;2000. p. 296–298.34. Gong YH. 13C NMR chemical shifts of natural organic compounds. Yunnan Science and Technology Publishing;1986. p. 252.35. Yang MH. Steroidal sapogenins of dioscorea. Chin Tradit Herb Drugs. 1981; 12:43–44.36. Xu CL, Chen HS, Tan XQ. Studies on the active constituents of Asparagi radix. Nat Prod Res. 2005; 17:128–130.37. Shen Y, Xu CL, Xuan WD, Li HL, Liu RH, Xu XK, Chen HS. A new furostanol saponin from Asparagus cochinchinensis. Arch Pharm Res. 2011; 34(10):1587–1591. PMID: 22076757.38. Li XN, Chu C, Cheng DP, Tong SQ, Yan JZ. Norlignans from Asparagus cochinchinensis. Nat Prod Commun. 2012; 7(10):1357–1358. PMID: 23157009.39. Zhu GL, Hao Q, Li RT, Li HZ. Steroidal saponins from the roots of Asparagus cochinchinensis. Chin J Nat Med. 2014; 12(3):213–217. PMID: 24702808.40. Kim H, Lee E, Lim T, Jung J, Lyu Y. Inhibitory effect of Asparagus cochinchinensis on tumor necrosis factor-alpha secretion from astrocytes. Int J Immunopharmacol. 1998; 20(4-5):153–162. PMID: 9730251.41. Lee do Y, Choo BK, Yoon T, Cheon MS, Lee HW, Lee AY, Kim HK. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009; 121(1):28–34. PMID: 18691647.42. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16(3):144–158.43. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999; 64:555–559.
Article44. Helaly FM, Soliman HSM, Soheir AD, Ahmed AA. Controlled release of migration of molluscicidal saponin from different types of polymers containing Calendula officinalis. Adv Polym tech. 2001; 20(4):305–311.45. Bae CJ, Lee JW, Bae HS, Shim SB, Jee SW, Lee SH, Lee CK, Hong JT, Hwang DY. Detection of allergenic compounds using an IL-4/luciferase/CNS-1 transgenic mice model. Toxicol Sci. 2011; 120(2):349–359. PMID: 21252390.
Article46. Choi SI, Lee HR, Goo JS, Kim JE, Nam SH, Hwang IS, Lee YJ, Prak SH, Lee HS, Lee JS, Jang IS, Son HJ, Hwang DY. Effects of Steaming Time and Frequency for Manufactured Red Liriope platyphylla on the Insulin Secretion Ability and Insulin Receptor Signaling Pathway. Lab Anim Res. 2011; 27(2):117–126. PMID: 21826171.47. Prussin C, Metcalfe DD. 4. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2003; 111:S486–S494. PMID: 12592295.
Article48. Kim YW, Zhao RJ, Park SJ, Lee JR, Cho IJ, Yang CH, Kim SG, Kim SC. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br J Pharmacol. 2008; 154(1):165–173. PMID: 18332856.49. Chiang YM, Lo CP, Chen YP, Wang SY, Yang NS, Kuo YH, Shyur LF. Ethyl caffeate suppresses NF-kappaB activation and its downstream inflammatory mediators, iNOS, COX-2, and PGE2 in vitro or in mouse skin. Br J Pharmacol. 2005; 146(3):352–363. PMID: 16041399.50. Kim H, Lee E, Lim T, Jung J, Lyu Y. Inhibitory effect of Asparagus cochinchinensis on tumor necrosis factor-alpha secretion from astrocytes. Int J Immunopharmacol. 1998; 20(4-5):153–162. PMID: 9730251.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial and Cytotoxic Activity of Di-(2-ethylhexyl) Phthalate and Anhydrosophoradiol-3-acetate Isolated from Calotropis gigantea (Linn.) Flower
- Dose dependence and durability of the therapeutic effects of Asparagus cochinchinensis fermented extract in an ovalbumin-challenged asthma model
- Hepatotoxicity and nephrotoxicity of saponin-enriched extract of Asparagus cochinchinensis in ICR mice
- Effect of Artemisia Capillaris Extract on the Growth of Food-Borne Pathogens
- Four amino acids as serum biomarkers for anti-asthma effects in the ovalbumin-induced asthma mouse model treated with extract of Asparagus cochinchinensis