Lab Anim Res.
2018 Sep;34(3):101-110. 10.5625/lar.2018.34.3.101.
Dose dependence and durability of the therapeutic effects of Asparagus cochinchinensis fermented extract in an ovalbumin-challenged asthma model
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- KMID: 2420822
- DOI: http://doi.org/10.5625/lar.2018.34.3.101
Abstract
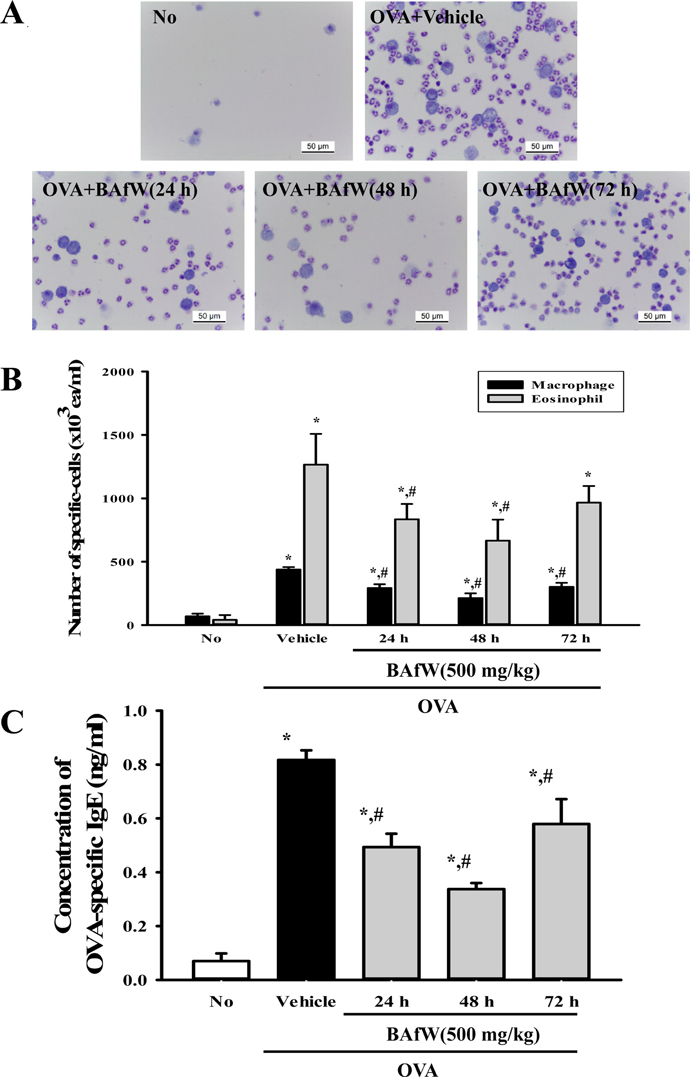
- The butanol extract of Asparagus cochinchinensis roots fermented with Weissella cibaria (BAfW) significantly suppressed the inflammatory response induced by lipopolysaccharide (LPS) treatment in RAW264.7 cells. To investigate the dose dependence and durability of BAfW on the anti-asthma effects, alterations in key parameters were measured in ovalbumin (OVA)-challenged Balb/c mice treated with the different doses of BAfW at three different time points. The number of immune cells, OVA-specific IgE level, thickness of respiratory epithelium and mucus score decreased significantly in a dose-dependent manner in response to treatment with 125 to 500 mg/kg BAfW (P < 0.05), although the highest level was detected in the 500 mg/kg treated group. Moreover, the decrease in these parameters was maintained from 24 to 48 h in the 500 mg/kg of BAfW treated group. At 72 h, the effects of BAfW on the number of immune cells, OVA-specific IgE level and thickness of respiratory epithelium partially disappeared. Overall, this study provides the first evidence that the anti-asthma effect of BAfW may reach the maximum level in OVA-challenged Balb/c mice treated with 500 mg/kg and that these effects can last for 48 h.
Keyword
MeSH Terms
Figure
Reference
-
1. Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014; 35(2):69–78.
Article2. Rosenberg JL. Antilipid agents may provide allergy protection. Ann Allergy Asthma Immunol. 2013; 110(1):1.
Article3. Porter PC, Yang T, Luong A, Delclos GL, Abramson SL, Kheradmand F, Corry DB. Proteinases as molecular adjuvants in allergic airway disease. Biochim Biophys Acta. 2011; 1810(11):1059–1065.
Article4. Walsh GM. Targeting airway inflammation: novel therapies for the treatment of asthma. Curr Med Chem. 2006; 13(25):3105–3111.5. Wise J. Corticosteroids for asthma may suppress growth in children in first year of treatment, researchers say. BMJ. 2014; 349:g4623.
Article6. Ciriaco M, Ventrice P, Russo G, Scicchitano M, Mazzitello G, Scicchitano F, Russo E. Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother. 2013; 4:Suppl 1. S94–S98.
Article7. Lee E, Haa K, Yook JM, Jin MH, Seo CS, Son KH, Kim HP, Bae KH, Kang SS, Son JK, Chang HW. Anti-asthmatic activity of an ethanol extract from Saururus chinensis. Biol Pharm Bull. 2006; 29(2):211–215.8. Jung WK, Choi I, Oh S, Park SG, Seo SK, Lee SW, Lee DS, Heo SJ, Jeon YJ, Je JY, Ahn CB, Kim JS, Oh KS, Kim YM, Moon C, Choi IW. Anti-asthmatic effect of marine red alga (Laurencia undulata) polyphenolic extracts in a murine model of asthma. Food Chem Toxicol. 2009; 47(2):293–297.9. Lee MY, Seo CS, Lee NH, Ha H, Lee JA, Lee H, Lee KY, Shin HK. Anti-asthmatic effect of schizandrin on OVA-induced airway inflammation in a murine asthma model. Int Immunopharmacol. 2010; 10(11):1374–1379.
Article10. Lee NH, Lee MY, Lee JA, Jung DY, Seo CS, Kim JH, Shin HK. Anti-asthmatic effect of Sanguisorba officinalis L. and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model. Int J Mol Med. 2010; 26(2):201–208.
Article11. Lee MY, Seo CS, Lee JA, Lee NH, Kim JH, Ha H, Zheng MS, Son JK, Shin HK. Anti-asthmatic effects of Angelica dahurica against ovalbumin-induced airway inflammation via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011; 49(4):829–837.12. Sung JE, Lee HA, Kim JE, Yun WB, An BS, Yang SY, Kim DS, Lee CY, Lee HS, Bae CJ, Hwang DY. Saponin-enriched extract of Asparagus cochinchinensis alleviates airway inflammation and remodeling in ovalbumin-induced asthma model. Int J Mol Med. 2017; 40(5):1365–1376.13. Vadnere GP, Gaud RS, Singhai AK. Evaluation of anti-asthmatic property of Solanum xanthocarpum flower extracts. Pharmacologyonline. 2008; 1:513–522.14. Tayade PM, Ghaisas MM, Jagtap SA, Dongre SH. Anti-asthmatic activity of methanolic extract of leaves of Tamarindus Indica Linn. J Pharm Res. 2009; 2(5):944–947.15. Deng YM, Xie QM, Zhang SJ, Yao HY, Zhang H. Anti-asthmatic effects of Perilla seed oil in the guinea pig in vitro and in vivo. Planta Med. 2007; 73(1):53–58.16. Lee DY, Choo BK, Yoon T, Cheon MS, Lee HW, Lee AY, Kim HK. Anti-inflammatory effects of Asparagus cochinchinensis extract in acute and chronic cutaneous inflammation. J Ethnopharmacol. 2009; 121(1):28–34.17. Sung JE, Lee HA, Kim JE, Go J, Seo EJ, Yun WB, Kim DS, Son HJ, Lee CY, Lee HS, Hwang DY. Therapeutic effect of ethyl acetate extract from Asparagus cochinchinensis on phthalic anhydride-induced skin inflammation. Lab Anim Res. 2016; 32(1):34–45.18. Jung KH, Choi HL, Pakr S, Lee G, Kim M, Min JK, Min BI, Bae H. The effects of the standardized herbal formula PM014 on pulmonary inflammation and airway responsiveness in a murine model of cockroach allergen-induced asthma. J Ethnopharmacol. 2014; 155(1):113–122.
Article19. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16:144–158.20. Lee HA, Song BR, Kim HR, Kim JE, Yun WB, Park JJ, Lee ML, Choi JY, Lee HS, Hwang DY. Butanol extracts of Asparagus cochinchinensis fermented with Weissella cibaria inhibit iNOS mediated COX 2 induction pathway and inflammatory cytokines in LPS stimulated RAW264.7 macrophage cells. Exp Ther Med. 2017; 14(5):4986–4994.21. Jung JY, Lee KY, Lee MY, Jung D, Cho ES, Son HY. Antioxidant and antiasthmatic effects of saucerneol D in a mouse model of airway inflammation. Int Immunopharmacol. 2011; 11(6):698–705.
Article22. Zhou E, Fu Y, Wei Z, Yu Y, Zhang X, Yang Z. Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia. 2014; 96:131–137.
Article23. Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 2010; 24(1):23–39.24. Kim D, Kim SH, Park EJ, Kang CY, Cho SH, Kim S. Anti-allergic effects of PG102, a water-soluble extract prepared from Actinidia arguta, in a murine ovalbumin-induced asthma model. Clin Exp Allergy. 2009; 39(2):280–289.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Four amino acids as serum biomarkers for anti-asthma effects in the ovalbumin-induced asthma mouse model treated with extract of Asparagus cochinchinensis
- MicroRNA-21 inhibition attenuates airway inflammation and remodelling by modulating the transforming growth factor β–Smad7 pathway
- Mega-dose vitamin C attenuated lung inflammation in mouse asthma model
- The New Phytoformula Containing Morus alba, Schizandra sinensis and Asparagus cochinchinensis Inhibits Lung Inflammation in vitro and in vivo
- IL-13 and STAT6 signaling involve in low dose lipopolysaccharide induced murine model of asthma