Nat Prod Sci.
2019 Sep;25(3):268-274. 10.20307/nps.2019.25.3.268.
Phytochemical Constituents of the Root Bark from Morus alba and Their Il-6 Inhibitory Activity
- Affiliations
-
- 1College of Pharmacy, Chosun University, Gwangju 61452, Republic of Korea. wooer@chosun.ac.kr
- 2Jiu Jiang University, College of Pharmacy and Life Science, Jiu Jiang 33200, China.
- KMID: 2459971
- DOI: http://doi.org/10.20307/nps.2019.25.3.268
Abstract
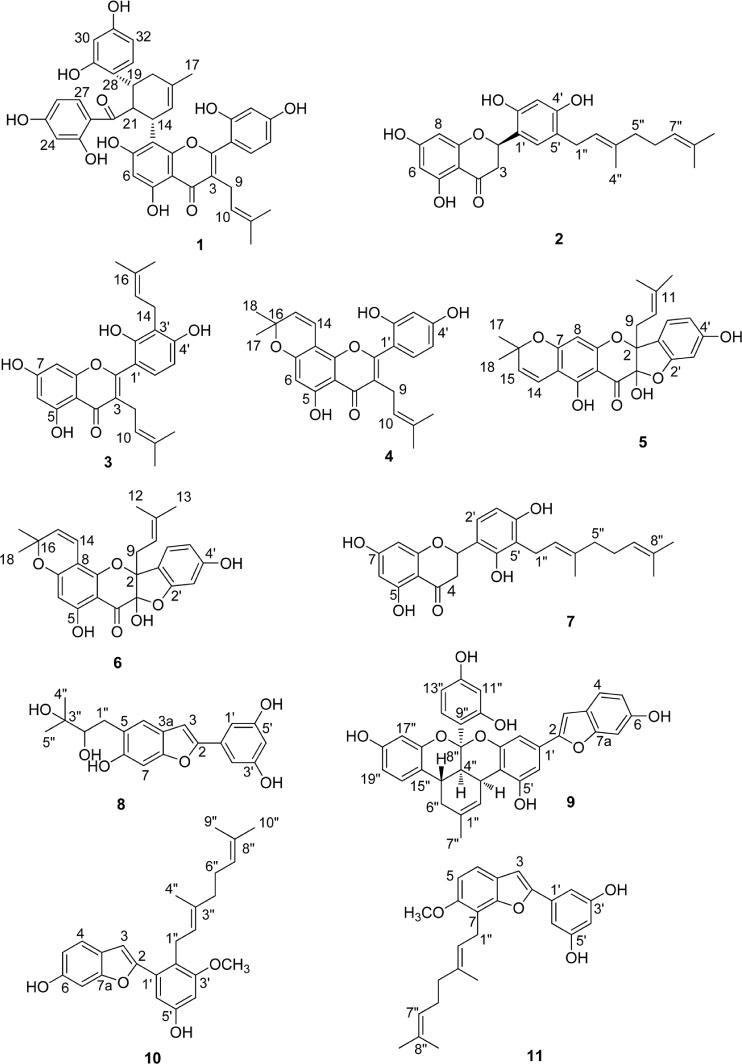
- Morus alba L., known as white mulberry, is a medicinal plant belongs to family Moraceae. It has long been used commonly in Ayurvedic for the treatment of lung-heat, cough, asthma, hematemesis, dropsy and hypertension. In the present study, seven prenylated flavonoids, along with four benzofuran compounds were isolated by means of repeated column chromatography. The structures of the known compounds were identified as kuwanon G (1), kuwanon E (2), kuwanon T (3), morusin (4), sanggenon A (5), sanggenon M (6), sanggenol A (7), moracin R (8), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11), by comparing their spectroscopic data with those reported in the literature. For these isolates, containing trace compounds, the inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells was examined. All isolated compounds (1 - 11) showed excellent inhibitory activity against IL-6 production in TNF-α stimulated MG-63 cells. Especially this study is first time to report that sanggenon A (5), sanggenon M (6), sanggenol A (7), mulberofuran G (9), mulberofuran A (10) and mulberofuran B (11) showed the inhibitory activity of IL-6 production. Our study suggested the possibility of anti-inflammatory regulation by compounds (1 - 11) isolated from M. alba.
MeSH Terms
Figure
Reference
-
1. Bae KH. The Medicinal Plants of Korea. Seoul: Kyo Hak Pub. Co.;2000. p. 73.2. Hano Y, Hirakura K, Nomura T, Terada S, Fukushima K. Planta Med. 1984; 50:127–130.3. Nomura T, Fukai T, Narita T. Heterocycle. 1980; 14:1943–1951.4. Nomura T, Fukai T. Planta Med. 1981; 42:79–88.5. Nomura T. Yakugaku Zassh. 2001; 121:535–556.6. Lim HJ, Jin HG, Woo ER, Lee SK, Kim HP. J Ethnopharmacol. 2013; 149:169–175.7. Kim BH, Chung EY, Ryu JC, Jung SH, Min KR, Kim Y. Arch Pharm Res. 2003; 26:306–311.8. Liu QH, Jeong JE, Choi EJ, Moon YH, Woo ER. Arch Pharm Res. 2006; 29:1109–1113.9. Chung MI, Lu CM, Huang PL, Lin CN. Phytochemistry. 1995; 40:1279–1282.10. Du J, He ZDm, Jiang RW, Ye WC, Xu HX, But PP. Phytochemistry. 2003; 62:1235–1238.11. Shen RC, Lin M. Phytochemistry. 2001; 57:1231–1235.12. Tan YX, Liu C, Zhang T, Chen RY, Yu DQ. Phytochem Lett. 2010; 3:57–61.13. Kapche GD, Fozing CD, Donfack JH, Fotso GW, Amadou D, Tchana AN, Bezabih M, Moundipa PF, Ngadjui BT, Abegaz BM. Phytochemistry. 2009; 70:216–221.14. Hano Y, Fukai T, Nomura T, Uzawa J, Fukushima K. Chem Pharm Bull. 1984; 32:1260–1263.15. Nomura T, Fukai T. Planta Med. 1981; 42:197–199.16. Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A, Tsunsawa S, Sakiyama F, Matsui H, Takahara Y, Taniguchi T, Kishimoto T. Nature. 1986; 324:73–76.17. Hirano T, Akira S, Taga T, Kishimoto T. Immunol Today. 1990; 11:443–449.18. Van Damme J, Opdenakker G, Simpson RJ, Rubira MR, Cayphas S, Vink A, Billiau A, Van Snick J. J Exp Med. 1987; 165:914–919.19. Qin J, Fan M, He J, Wu XD, Peng LY, Su J, Cheng X, Li Y, Kong LM, Li RT, Zhao QS. Nat Prod Res. 2015; 29:1711–1718.20. Wu YX, Kim YJ, Kwon TH, Tan CP, Son KH, Kim T. Nat Prod Res. 2018; 23:1–4.21. Liu XX, Zhang XW, Wang K, Wang XY, Ma WL, Cao W, Mo D, Sun Y, Li XQ. Toxicol Appl Pharmacol. 2018; 341:56–63.22. Jung HW, Kang SY, Kang JS, Kim AR, Woo ER, Park YK. Phytother Res. 2014; 28:1713–1719.23. Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP. Biochem Pharmacol. 2001; 62:1185–1191.24. Tseng TH, Lin WL, Chang CK, Lee KC, Tung SY, Kuo HC. Cell Physiol Biochem. 2018; 51:1376–1388.25. Dat NT, Binh PT, Quynh LTP, Huong HT, Minh CV. . Immunopharmacol Immunotoxicol. 2012; 34:84–88.26. Jung JW, Ko WM, Park JH, Seo KH, Oh EJ, Lee DY, Lee DS, Kim YC, Lim DW, Han D, Baek NI. Arch Pharm Res. 2015; 38:2066–2075.27. Shim SY, Sung SH, Lee M. Int Immunopharmacol. 2018; 58:117–124.28. Kang J, Chen RY, Yu DQ. Planta Med. 2006; 72:52–59.29. Sohn HY, Son KH, Kwon CS, Kwon GS, Kang SS. Phytomedicine. 2004; 11:666–672.30. Geng CA, Ma YB, Zhang XM, Yao SY, Xue DQm. J Agric Food Chem. 2012; 60:8197–8202.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Morus alba L. and Natural Products Including Morusin on In Vivo Secretion and In Vitro Production of Airway MUC5AC Mucin
- Efficacy and safety of a botanical shampoo containing Morus alba root extract in Republic of Korea: a single-center, open-label, pilot study in mild to moderate non-scarring alopecia of the scalp
- The New Phytoformula Containing Morus alba, Schizandra sinensis and Asparagus cochinchinensis Inhibits Lung Inflammation in vitro and in vivo
- Enhancing the Angiogenic and Proliferative Capacity of Dermal Fibroblasts with Mulberry ( Morus alba. L) Root Extract
- Inhibitory Effect of Polysaccharide Fraction from Cortex Mori on Compound 48/80-Induced Mast Cell Activation