J Breast Cancer.
2010 Mar;13(1):14-18. 10.4048/jbc.2010.13.1.14.
Immunologic Response to Mistletoe Extract (Viscum album L.) after Conventional Treatment in Patients with Operable Breast Cancer
- Affiliations
-
- 1Department of Surgery, Korea University College of Medicine, Seoul, Korea. kujwbae@korea.ac.kr
- 2Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- KMID: 2286554
- DOI: http://doi.org/10.4048/jbc.2010.13.1.14
Abstract
- PURPOSE
To reduce the side effects and improve the effectiveness of standard chemoradiation therapy, many complementary or alternative medicines have been tried. However, little is known about its immunologic effects in breast cancer patients. The aim of this study was to assess the immunologic effects of mistletoe extract (Viscum album L., VAE) in patients with early breast cancer after surgery followed by standard adjuvant chemoradiation therapy.
METHODS
A total 20 patients with early breast cancer treated with breast conserving surgery followed conventional chemoradiation therapy. Ten of these patients received subcutaneous injections of VAE for 7 weeks. IL-2, IL-4, IL-6, IL-10, TGF-beta, and IFN-gamma levels in serum samples were measured in all patients.
RESULTS
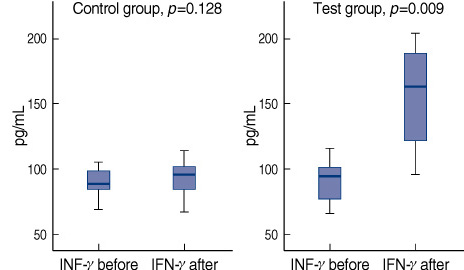
The concentrations of IL-2, IL-4, IL-10, and TGF-beta were not significantly changed between before and after VAE treatment in both test and control group. The concentration of IL-6 in the test group was increased from 8.19+/-1.75 pg/mL to 9.86+/-1.46 pg/mL after treatment (p=0.013). The concentration of IFN-gamma in the test group was remarkably increased from 91.76+/-17.16 pg/mL to 167.42+/-66.61 pg/mL after treatment (p=0.009).
CONCLUSION
Significant increases in the concentration of IL-6 and IFN-gamma were observed after VAE treatment. These results suggest that VAE treatment can stimulate immune responses, especially cell-mediated immunity in immune-compromised patients received the chemoradiation for breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Ernst E, Schmidt K, Steuer-Vogt MK. Mistletoe for cancer? A systematic review of randomised clinical trials. Int J Cancer. 2003. 107:262–267.2. Bussing A. Immune modulation using mistletoe (Viscum album L.) extracts Iscador. Arzneimittelforschung. 2006. 56:508–515.3. Kienle GS, Berrino F, Büssing A, Portalupi E, Rosenzweig S, Kiene H. Mistletoe in cancer: a systematic review on controlled clinical trials. Eur J Med Res. 2003. 8:109–119.4. Kienle GS, Kiene H. Complementary cancer therapy: a systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur J Med Res. 2007. 12:103–119.5. Lyu SY, Park WB. Mistletoe lectin (Viscum album coloratum) modulates proliferation and cytokine expressions in murine splenocytes. J Biochem Mol Biol. 2006. 39:662–670.
Article6. Sulizeanu D. Immunosuppressive factors in human cancer. Adv Cancer Res. 1993. 60:247–267.7. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989. 7:145–173.
Article8. Hajto T, Hostanska K, Gabius HJ. Modulatory potency of the beta-galactoside-specific lectin from Mistletoe Extract (Iscador) on the host defense system in vivo in rabbits and patients. Cancer Res. 1989. 49:4803–4808.9. Kovacs E. Serum levels of IL-12 and the production of IFN-gamma, IL-2 and IL-4 by peripheral blood mononuclear cells (PBMC) in cancer patients treated with Viscum album extract. Biomed Pharmacother. 2000. 54:305–310.
Article10. Gardin NE. Immunological response to mistletoe (Viscum album L.) in cancer patients: a four-case series. Phytother Res. 2009. 23:407–411.
Article11. Yokoe T, Iino Y, Morishita Y. Trends of IL-6 and IL-8 levels in patients with recurrent breast cancer: preliminary report. Breast Cancer. 2000. 7:187–190.
Article12. Knüpfer H, Preiss R. Significance of interleukin-6 in breast cancer (review). Breast Cancer Res Treat. 2007. 102:129–135.
Article13. Kurebayashi J. Regulation of interleukin-6 secretion from breast cancer cells and its clinical implications. Breast Cancer. 2000. 7:124–129.
Article14. Mullen CA, Coale MM, Levy AT, Stetler-Stevenson WG, Liotta LA, Blaese RM. Fibrosarcoma cells transduced with the IL-6 gene exhibited reduced tumorigenicity, increased immunogenicity, and decreased metastatic potential. Cancer Res. 1992. 52:6020–6024.15. Sun WH, Kreisle RA, Phillips AW, Ershler WB. In vivo and in vitro characteristics of interleukin 6-transfected B16 melanoma cells. Cancer Res. 1992. 52:5412–5415.16. Xiao B, Jing B, Zhang YL, Zhou DY, Zhang WD. Tumor growth inhibition effect of hIL-6 on colon cancer cells transfected with the target gene by retroviral vector. World J Gastroenterol. 2000. 6:89–92.
Article17. Luger TA, Krutmann J, Kirnbauer R, Urbanski A, Schwarz T, Klappacher G, et al. IFN-beta 2/IL-6 augments the activity of human natural killer cells. J Immunol. 1989. 143:1206–1209.18. Ishiguro H, Kishimoto T, Furuya M, Nagai Y, Watanabe T, Ishikura H. Tumor-derived interleukin (IL)-6 induced anti-tumor effect in immune-compromised hosts. Cancer Immunol Immunother. 2005. 54:1191–1199.
Article19. Elluru SR, van Huyen JP, Delignat S, Kazatchkine MD, Friboulet A, Kaveri SV, et al. Induction of maturation and activation of human dendritic cells: a mechanism underlying the beneficial effect of Viscum album as complimentary therapy in cancer. BMC Cancer. 2008. 8:161.20. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007. 96:41–101.21. Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001. 410:1107–1111.
Article22. Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE Jr. Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A. 1996. 93:7673–7678.
Article23. Schreiber RD, Celada A, Buchmeier N. The role of interferon-gamma in the induction of activated macrophages. Ann Inst Pasteur Immunol. 1986. 137:203–206.
Article24. Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000. 30:985–992.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunological Activities of Korean Mistletoe Extract ( Viscum album coloratum ; KM - 110 )
- Chemical Pleurodesis Using Viscum album Extract in Gorham Disease Complicated with Chylothorax
- Immumoadjuvant Activitiy of Korean Mistletoe Extract ( Viscum album coloratum ) to Enhance Humoral and Cellular Immune Response
- Chemical Pleurodesis Using Doxycycline and Viscum album Extract
- Chemical Pleurodesis Using a Viscum album Extract in an Infant with Postoperative Chylothorax: A Case Report