J Breast Cancer.
2011 Jun;14(2):88-95. 10.4048/jbc.2011.14.2.88.
Overexpression of MMP-9 and HIF-1alpha in Breast Cancer Cells under Hypoxic Conditions
- Affiliations
-
- 1Gangnam Yeil Clinic, Seoul, Korea.
- 2Department of Surgery, Eulji University School of Medicine, Seoul, Korea.
- 3Department of Surgery, Kyung Hee University School of Medicine, Seoul, Korea. jeonguni@khu.ac.kr
- KMID: 2286506
- DOI: http://doi.org/10.4048/jbc.2011.14.2.88
Abstract
- PURPOSE
Hypoxia, which is a loss of oxygen in tissues, is a common condition in solid tumors due to the tumor outgrowing existing vasculature. Under hypoxic conditions, hypoxia-inducible factor (HIF)-1alpha rapidly accumulates and transactivates hundreds of genes, such as matrix metalloproteinases (MMPs). MMPs contribute to invasion and metastasis of tumor cells by degrading the surrounding basement membrane and extracellular matrix barriers, which enables the easy migration and spread of cancer cells. We examined whether hypoxia increases tumor cell invasion, and whether increased invasiveness was due to HIF-1alpha and MMP-9 expression.
METHODS
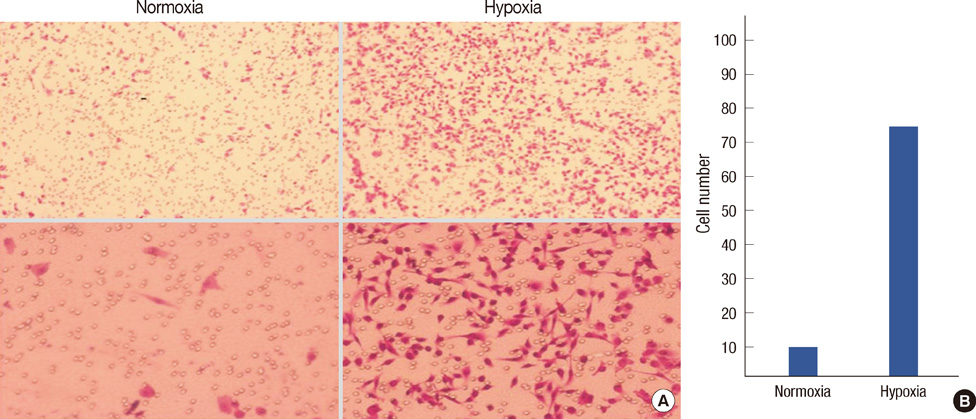
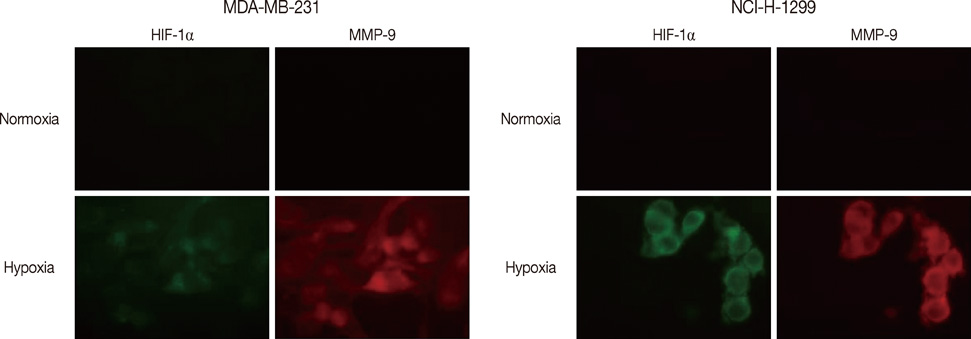
Transwell invasion assays were performed to demonstrate whether hypoxia enhance tumor invasion by use of MDA-MB-231 breast cancer cells. An immunofluorescence assay was used to demonstrate expression of HIF-1alpha and MMP-9 under hypoxic conditions. Luciferase and ChiP assays were performed to demonstrate that MMP-9 promoter activity was regulated by HIF-1alpha.
RESULTS
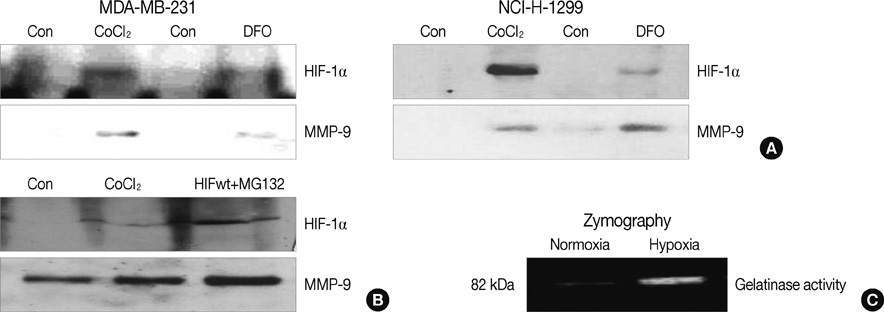
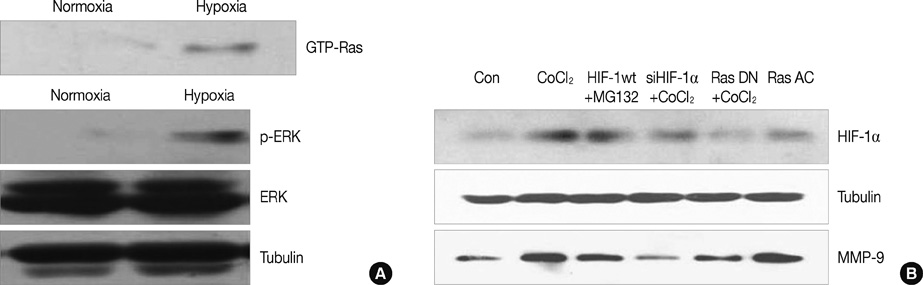
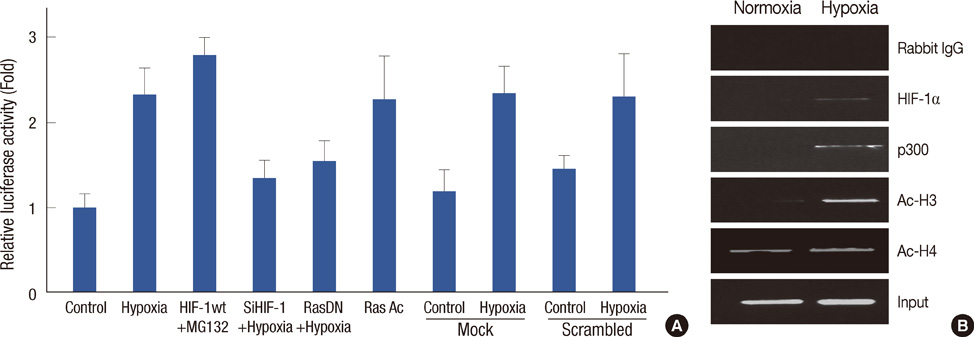
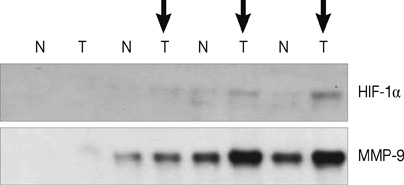
HIF-1alpha was stabilized under hypoxic conditions and stimulated MMP-9 expression, which affected the tumor invasiveness of breast cancer cells. HIF-1alpha transactivated the MMP-9 promoter by forming a transcriptional unit with p300, thus increasing expression of MMP-9 transcripts. Zymography indicated that MMP-9 had more gelatinase activity under hypoxic conditions than normoxic conditions. Furthermore, the small GTPase Ras was also activated in response to hypoxia, which then aids stabilization of HIF-1alpha, and in turn upregulates MMP-9 expression. We also demonstrate that MMP-9 is upregulated concurrently with HIF-1alpha in tumor tissues from patients with breast cancer.
CONCLUSION
These results suggest that HIF-1alpha promotes cell invasion through a MMP-9-dependent mechanism and that future antitumor agents could be used to target HIF-1alpha and MMP-9.
Keyword
MeSH Terms
-
Anoxia
Antineoplastic Agents
Basement Membrane
Breast
Breast Neoplasms
Extracellular Matrix
Fluorescent Antibody Technique
Gelatinases
GTP Phosphohydrolases
Humans
Luciferases
Matrix Metalloproteinases
Neoplasm Metastasis
Oxygen
Antineoplastic Agents
GTP Phosphohydrolases
Gelatinases
Luciferases
Matrix Metalloproteinases
Oxygen
Figure
Reference
-
1. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002. 2:38–47.2. Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003. 94:1021–1028.
Article3. Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004. 9:Suppl 5. 10–17.
Article4. Vaupel P, Kelleher DK, Höckel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy. Semin Oncol. 2001. 28:2 Suppl 8. 29–35.
Article5. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003. 3:721–732.
Article6. Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda). 2004. 19:176–182.
Article7. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001. 292:464–468.
Article8. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001. 292:468–472.
Article9. Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000. 106:809–812.
Article10. Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002. 8:4 Suppl. S62–S67.
Article11. Freije JM, Balbín M, Pendás AM, Sánchez LM, Puente XS, López-Otín C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003. 532:91–107.
Article12. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000. 18:1135–1149.
Article13. Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999. 59:5830–5835.14. Duffy MJ, Maguire TM, Hill A, McDermott E, O'Higgins N. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000. 2:252–257.
Article15. Jones JL, Glynn P, Walker RA. Expression of MMP-2 and MMP-9, their inhibitors, and the activator MT1-MMP in primary breast carcinomas. J Pathol. 1999. 189:161–168.
Article16. Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J Neurooncol. 2006. 78:233–247.
Article17. Bos R, van Diest PJ, van der Groep P, Shvarts A, Greijer AE, van der Wall E. Expression of hypoxia-inducible factor-1alpha and cell cycle proteins in invasive breast cancer are estrogen receptor related. Breast Cancer Res. 2004. 6:R450–R459.18. Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, et al. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003. 97:1573–1581.
Article19. Gruber G, Greiner RH, Hlushchuk R, Aebersold DM, Altermatt HJ, Berclaz G, et al. Hypoxia-inducible factor 1 alpha in high-risk breast cancer: an independent prognostic parameter? Breast Cancer Res. 2004. 6:R191–R198.
Article20. Pritchard SC, Nicolson MC, Lloret C, McKay JA, Ross VG, Kerr KM, et al. Expression of matrix metalloproteinases 1, 2, 9 and their tissue inhibitors in stage II non-small cell lung cancer: implications for MMP inhibition therapy. Oncol Rep. 2001. 8:421–424.
Article21. Zhang X, Zhu S, Luo G, Zheng L, Wei J, Zhu J, et al. Expression of MMP-10 in lung cancer. Anticancer Res. 2007. 27(4C):2791–2795.22. Hanemaaijer R, Verheijen JH, Maguire TM, Visser H, Toet K, McDermott E, et al. Increased gelatinase-A and gelatinase-B activities in malignant vs. benign breast tumors. Int J Cancer. 2000. 86:204–207.
Article23. Incorvaia L, Badalamenti G, Rini G, Arcara C, Fricano S, Sferrazza C, et al. MMP-2, MMP-9 and activin A blood levels in patients with breast cancer or prostate cancer metastatic to the bone. Anticancer Res. 2007. 27(3B):1519–1525.24. Canning MT, Postovit LM, Clarke SH, Graham CH. Oxygen-mediated regulation of gelatinase and tissue inhibitor of metalloproteinases-1 expression by invasive cells. Exp Cell Res. 2001. 267:88–94.
Article25. Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003. 63:1138–1143.26. Hernández-Alcoceba R, del Peso L, Lacal JC. The Ras family of GTPases in cancer cell invasion. Cell Mol Life Sci. 2000. 57:65–76.
Article27. Nguyen M, McCombs MM, Ghandehari S, Kim A, Wang H, Barsky SH, et al. An update on core needle biopsy for radiologically detected breast lesions. Cancer. 1996. 78:2340–2345.
Article28. Klimberg VS. Advances in the diagnosis and excision of breast cancer. Am Surg. 2003. 69:11–14.29. Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001. 21:3995–4004.
Article30. Giatromanolaki A, Koukourakis MI, Simopoulos C, Polychronidis A, Gatter KC, Harris AL, et al. c-erbB-2 related aggressiveness in breast cancer is hypoxia inducible factor-1alpha dependent. Clin Cancer Res. 2004. 10:7972–7977.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Hypoxia Inducible Factor-1alpha by Desferrioxamine Induces Radioresistance in Mouse Hepatoma Cell Line
- CaMKII Inhibitor KN-62 Blunts Tumor Response to Hypoxia by Inhibiting HIF-1alpha in Hepatoma Cells
- Immunohistochemical Expression and Prognostic Value of VEGF, HIF-1alpha, EGFR in Non-Small Cell Lung Cancer
- HIF-1alpha: a Valid Therapeutic Target for Tumor Therapy
- Expression of MMP-2, HIF-1alpha and VEGF in Colon Adenoma and Colon Cancer