Korean J Physiol Pharmacol.
2010 Oct;14(5):331-336. 10.4196/kjpp.2010.14.5.331.
CaMKII Inhibitor KN-62 Blunts Tumor Response to Hypoxia by Inhibiting HIF-1alpha in Hepatoma Cells
- Affiliations
-
- 1Department of Physiology, Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul 110-799, Korea. lee12042@snu.ac.kr
- KMID: 2071702
- DOI: http://doi.org/10.4196/kjpp.2010.14.5.331
Abstract
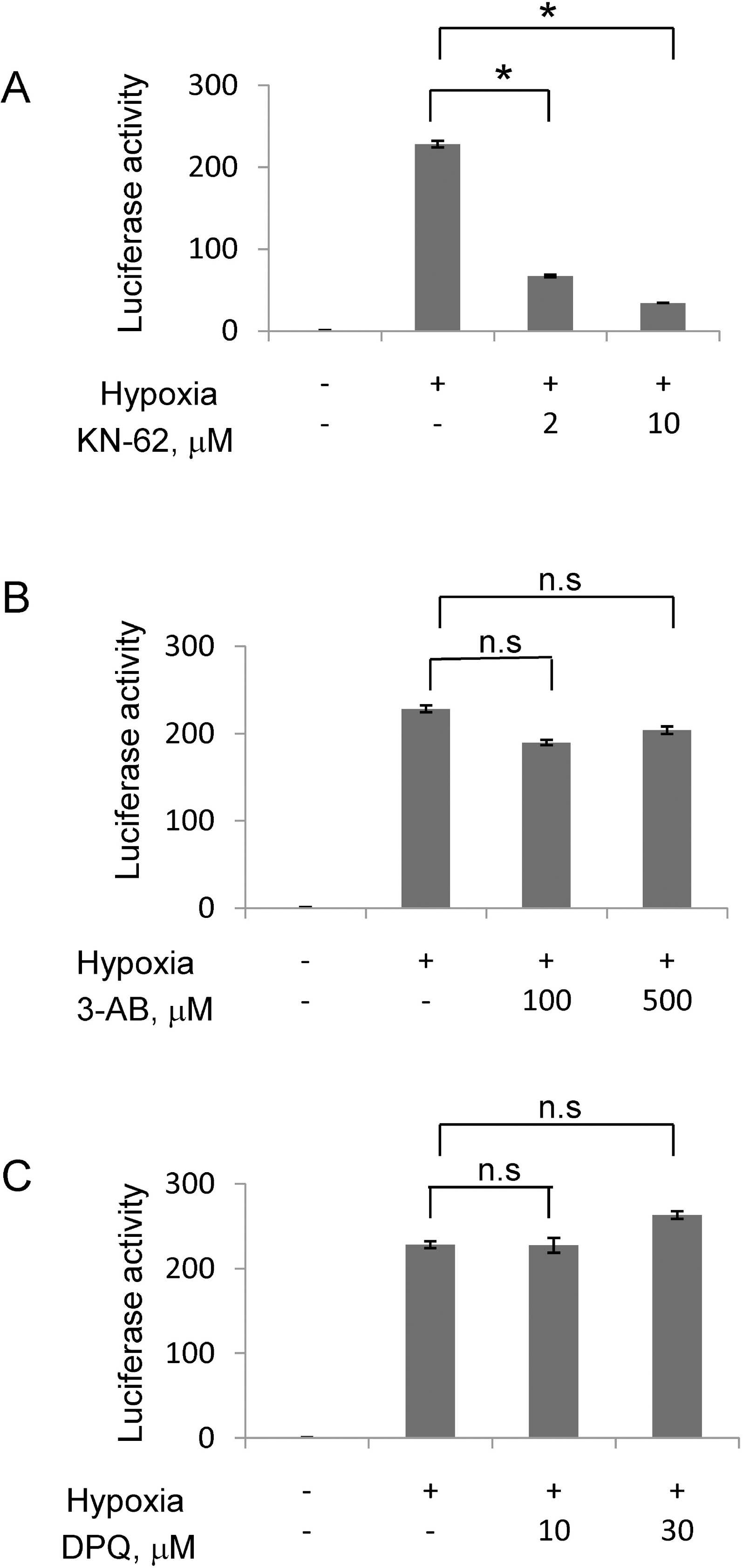
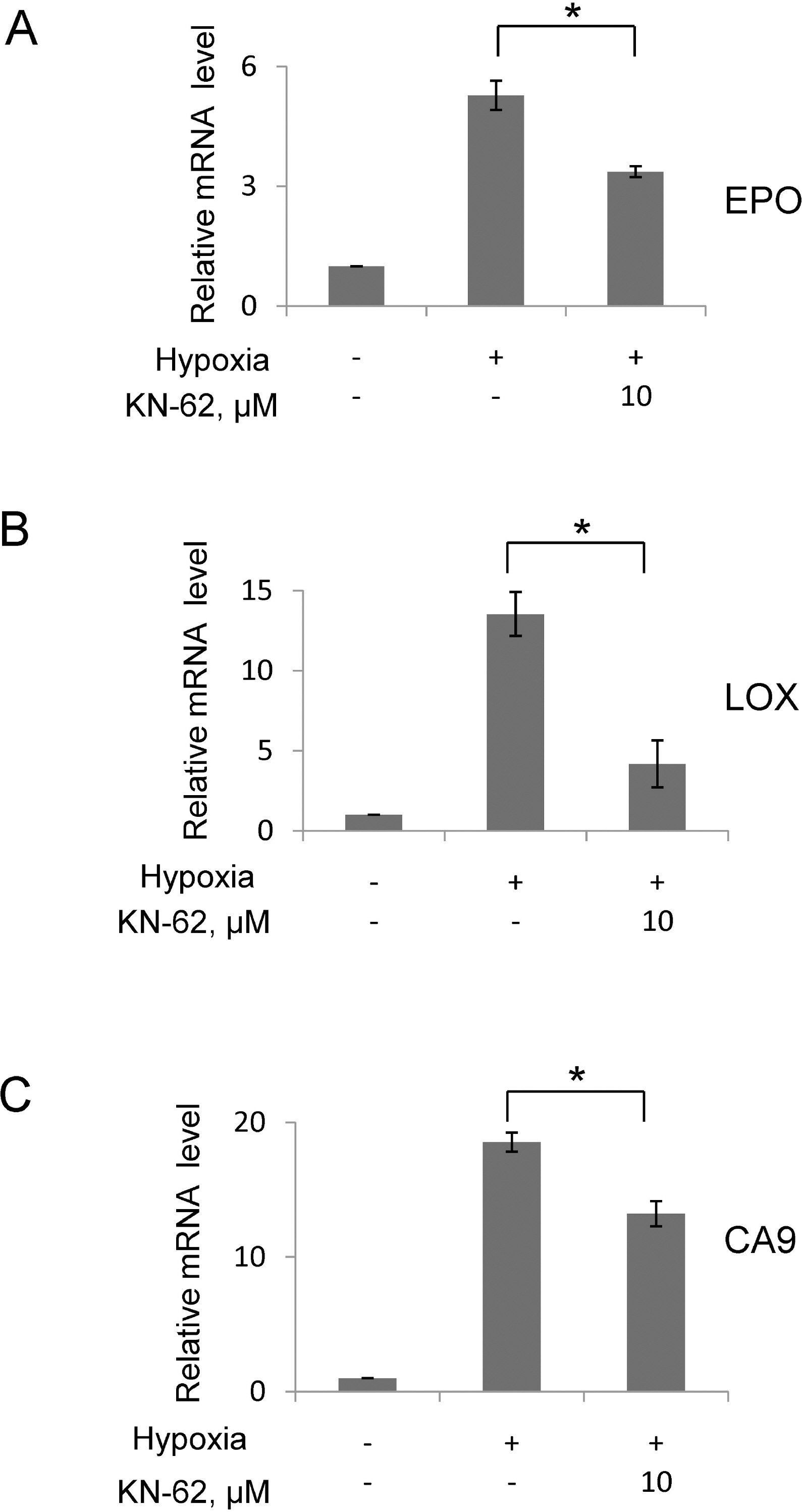
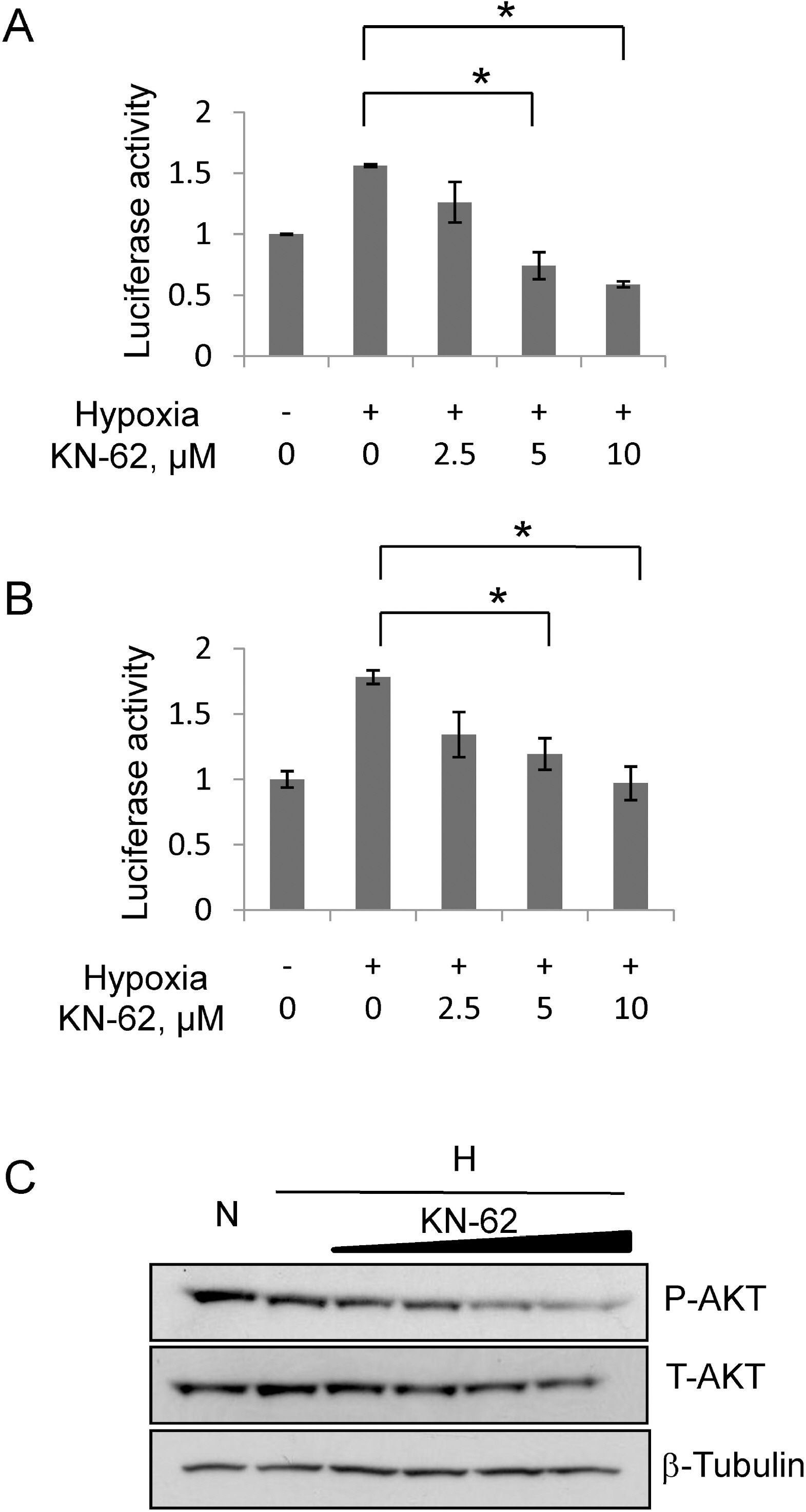
- In rapidly growing tumors, hypoxia commonly develops due to the imbalance between O2 consumption and supply. Hypoxia Inducible Factor (HIF)-1alpha is a transcription factor responsible for tumor growth and angiogenesis in the hypoxic microenvironment; thus, its inhibition is regarded as a promising strategy for cancer therapy. Given that CamKII or PARP inhibitors are emerging anticancer agents, we investigated if they have the potential to be developed as new HIF-1alpha-targeting drugs. When treating various cancer cells with the inhibitors, we found that a CamKII inhibitor, KN-62, effectively suppressed HIF-1alpha specifically in hepatoma cells. To examine the effect of KN-62 on HIF-1alpha-driven gene expression, we analyzed the EPO-enhancer reporter activity and mRNA levels of HIF-1alpha downstream genes, such as EPO, LOX and CA9. Both the reporter activity and the mRNA expression were repressed by KN-62. We also found that KN-62 suppressed HIF-1alpha by impairing synthesis of HIF-1alpha protein. Based on these results, we propose that KN-62 is a candidate as a HIF-1alpha-targeting anticancer agent.
Keyword
MeSH Terms
-
1-(5-Isoquinolinesulfonyl)-2-Methylpiperazine
Anoxia
Antineoplastic Agents
Calcium-Calmodulin-Dependent Protein Kinase Type 2
Carcinoma, Hepatocellular
Gene Expression
RNA, Messenger
Transcription Factors
1-(5-Isoquinolinesulfonyl)-2-Methylpiperazine
Antineoplastic Agents
Calcium-Calmodulin-Dependent Protein Kinase Type 2
RNA, Messenger
Transcription Factors
Figure
Cited by 1 articles
-
Regulation of Ca2+ Signaling in Pulmonary Hypertension
Amy L. Firth, Jun Yeon Won, Won Sun Park
Korean J Physiol Pharmacol. 2013;17(1):1-8. doi: 10.4196/kjpp.2013.17.1.1.
Reference
-
References
1. Hockel M, Vaupel P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. J Natl Cancer Inst. 2001; 93:266–276.
Article2. Semenza G, Wang G. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992; 12:5447–5454.
Article3. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O-2-regulated prolyl hydroxylation. Science. 2001; 292:468–472.4. Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998; 95:7987–7992.5. Berra E, Ginouvès A, Pouysségur J. The hypoxia-inducible-factor hydroxylases bring fresh air into hypoxia signalling. EMBO Reports. 2006; 7:41–45.
Article6. Pugh C, O'Rourke J, Nagao M, Gleadle J, Ratcliffe P. Activation of hypoxia-inducible factor-1; definition of regulatory domains within the alpha subunit. J Biol Chem. 1997; 272:11205.7. Arany Z, Huang L, Eckner R, Bhattacharya S, Jiang C, Goldberg M, Bunn H, Livingston D. An essential role for p300/CBP in the cellular response to hypoxia. Proc Natl Acad Sci USA. 1996; 93:12969.
Article8. Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza G, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem. 2002; 277:27975.
Article9. Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of Hypoxia-Inducible Factor 1alpha Expression and Function by the Mammalian Target of Rapamycin. Mol Cell Biol. 2002; 22:7004–7014.10. Ducibella T, Schultz RM, Ozil JP. Role of calcium signals in early development. Semin Cell Dev Biol. 2006; 17:324–332.
Article11. Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006; 97:56–70.
Article12. Minami H, Inoue S, Hidaka H. The Effect of KN-62, Ca2+/Calmodulin Dependent Protein Kinase II Inhibitor on Cell Cycle. Biochem Biophys Res Commun. 1994; 199:241–248.13. Tombes R, Grant S, Westin E, Krystal G. G1 cell cycle arrest and apoptosis are induced in NIH 3T3 cells by KN-93, an inhibitor of CaMK-II (the multifunctional Ca2+/CaM kinase). Cell Growth Differ. 1995; 6:1063–1070.14. Hui AS, Bauer AL, Striet JB, Schnell PO, Czyzyk-Krzeska MF. Calcium signaling stimulates translation of HIF-1 alpha during hypoxia. FASEB J. 2006; 20:466–475.15. Youn H-D, Chatila TA, Liu JO. Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J. 2000; 19:4323–4331.
Article16. Meissner JD, Umeda PK, Chang KC, Gros G, Scheibe RJ. Activation of the β myosin heavy chain promoter by MEF-2D, MyoD, p300, and the calcineurin/NFATc1 pathway. J Cell Physiol. 2007; 211:138–148.17. Rhun YL, Kirkland JB, Shah GM. Cellular Responses to DNA Damage in the Absence of Poly (ADP-ribose) Polymerase. Biochem Biophys Res Commun. 1998; 245:1–10.18. Ermak G, Davies KJ. Calcium and oxidative stress: from cell signaling to cell death. Mol Immunol. 2002; 38:713–721.
Article19. Virag L, Scott GS, Antal-Szalmas P, O'Connor M, Ohshima H, Szabo C. Requirement of intracellular calcium mobilization for peroxynitrite-induced poly (ADP-ribose) synthetase activation and cytotoxicity. Mol Pharmacol. 1999; 56:824–833.20. Martin-Oliva D, Aguilar-Quesada R, O'Valle F, Muñoz-Gámez JA, Martínez-Romero R, Garcíadel Moral R, Ruiz de Almodóvar JM, Villuendas R, Piris MA, Oliver FJ. Inhibition of poly (ADP-Ribose) polymerase modulates tumor-related gene expression, including hypoxia-inducible factor-1 activation, during skin carcinogenesis. Cancer Research. 2006; 66:5744–5756.21. Martínez-Romero R, Martínez-Lara E, Aguilar-Quesada R, Peralta A, Oliver FJ, Siles E. PARP-1 modulates deferoxamine-induced HIF-1alpha accumulation through the regulation of nitric oxide and oxidative stress. J Cell Biochem. 2008; 104:2248–2260.22. Martínez-Romero R, Cañuelo A, Martínez-Lara E, Oliver FJ, Cárdenas S, Siles E. Poly (ADP-ribose) polymerase-1 modulation of in vivo response of brain hypoxia-inducible factor-1 to hypoxia/reoxygenation is mediated by nitric oxide and factor inhibiting HIF. J Neurochem. 2009; 111:150–159.23. Elser M, Borsig L, Hassa PO, Erener S, Messner S, Valovka T, Keller S, Gassmann M, Hottiger MO. Poly (ADP-Ribose) polymerase 1 promotes tumor cell survival by coactivating hypoxia-inducible factor-1-dependent gene expression. Molecular Cancer Research. 2008; 6:282–290.24. Chun YS, Choi E, Yeo EJ, Lee JH, Kim MS, Park JW. A new HIF-1 alpha variant induced by zinc ion suppresses HIF-1-mediated hypoxic responses. J Cell Sci. 2001; 114:4051–4061.
Article25. Lee KH, Li M, Michalowski AM, Zhang X, Liao H, Chen L, Xu Y, Wu X, Huang J. A genomewide study identifies the Wnt signaling pathway as a major target of p53 in murine embryonic stem cells. Proc Natl Acad Sci U S A. 2010; 107:69–74.
Article26. Shin HW, Cho CH, Kim TY, Park JW. Sunitinib deregulates tumor adaptation to hypoxia by inhibiting HIF-1alpha synthesis in HT-29 colon cancer cells. Biochem Biophys Res Commun. 2010; 398:205–211.27. Lang KJD, Kappel A, Goodall GJ. Hypoxia-inducible Factor1alpha mRNA Contains an Internal Ribosome Entry Site That Allows Efficient Translation during Normoxia and Hypoxia. Mol Biol Cell. 2002; 13:1792–1801.28. Arsham AM, Plas DR, Thompson CB, Simon MC. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1 alpha nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002; 277:15162–15170.29. Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling–an overview. Semin Cell Dev Biol. 2001; 12:3–10.
Article30. Howe CJ, LaHair MM, McCubrey JA, Franklin RA. Redox Regulation of the Calcium/Calmodulin-dependent Protein Kinases. J Biol Chem. 2004; 279:44573–44581.
Article31. Yuan G, Nanduri J, Bhasker C, Semenza G, Prabhakar N. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005; 280:4321–4328.32. Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: Involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008; 217:674–685.33. Westra J, Brouwer E, Bos R, Posthumus MD, Meer BD-VD, Kallenberg CGM, Limburg PC. Regulation of Cytokine-Induced HIF-1alpha; Expression in Rheumatoid Synovial Fibroblasts. Ann N Y Acad Sci. 2007; 1108:340–348.34. Westra J, Brouwer E, Bouwman E, Meer BD-vd, Posthumus MD, Leeuwen MAv, Limburg PC, Ueda Y, Kallenberg CGM. Role for CaMKII Inhibition in Rheumatoid Arthritis. Ann N Y Acad Sci. 2009; 1173:706–711.
Article35. Westra J, Brouwer E, van Roosmalen IA, Doornbos-van der Meer B, van Leeuwen MA, Posthumus MD, Kallenberg CG. Expression and regulation of HIF-1alpha in macrophages under inflammatory conditions; significant reduction of VEGF by CaMKII inhibitor. BMC Musculoskelet Disord. 2010; 11:61.
Article36. Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: Worldwide incidence and trends. Gastroenterology. 2004; 127:S5–S16.
Article37. Jee SH, Ohrr H, Sull JW, Samet JM. Cigarette Smoking, Alcohol Drinking, Hepatitis B, and Risk for Hepatocellular Carcinoma in Korea. J Natl Cancer Inst. 2004; 96:1851–1856.
Article38. Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005; 5:876–885.
Article39. Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer Science. 2003; 94:15–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Hypoxia Inducible Factor-1alpha by Desferrioxamine Induces Radioresistance in Mouse Hepatoma Cell Line
- Overexpression of MMP-9 and HIF-1alpha in Breast Cancer Cells under Hypoxic Conditions
- The Effect of HIF-1alpha siRNA on Growth and Chemosensitivity of Mia-paca Cell Line
- Phospholipase D2 promotes degradation of hypoxia-inducible factor-1alpha independent of lipase activity
- The Relationship between Expression of Hypoxia Inducible Factor-1alpha or Vascular Endothelial Growth Factor and Histopathological Characteristics in Human Transitional Bladder Cancer