J Breast Cancer.
2013 Sep;16(3):291-299. 10.4048/jbc.2013.16.3.291.
Silencing of Fanconi Anemia Complementation Group F Exhibits Potent Chemosensitization of Mitomycin C Activity in Breast Cancer Cells
- Affiliations
-
- 1Department of Pharmacology, China Medical University, Shenyang, China. weiminjiecmu@163.com
- 2Institute of Pathology and Pathophysiology, China Medical University, Shenyang, China.
- KMID: 2286380
- DOI: http://doi.org/10.4048/jbc.2013.16.3.291
Abstract
- PURPOSE
Fanconi anemia complementation group F (FANCF) is a key factor to maintaining the function of Fanconi anaemia/BRCA (FA/BRCA) pathway, a DNA-damage response pathway. However, the functional role of FANCF in breast cancer has not been elucidated. In the present study, we evaluated the chemosensitization effect of FANCF in breast cancer cells.
METHODS
We performed specific knockdown of the endogenous FANCF in breast cancer cells by transfecting the cells with an FANCF short hairpin RNA (shRNA) vector. Cell viability was measured with a Cell Counting Kit-8, and DNA damage was assessed with the alkaline comet assay. The apoptosis, cell cycle, and drug accumulation were measured by flow cytometric analysis. Protein expression levels were determined by Western blot analysis, using specific antibodies.
RESULTS
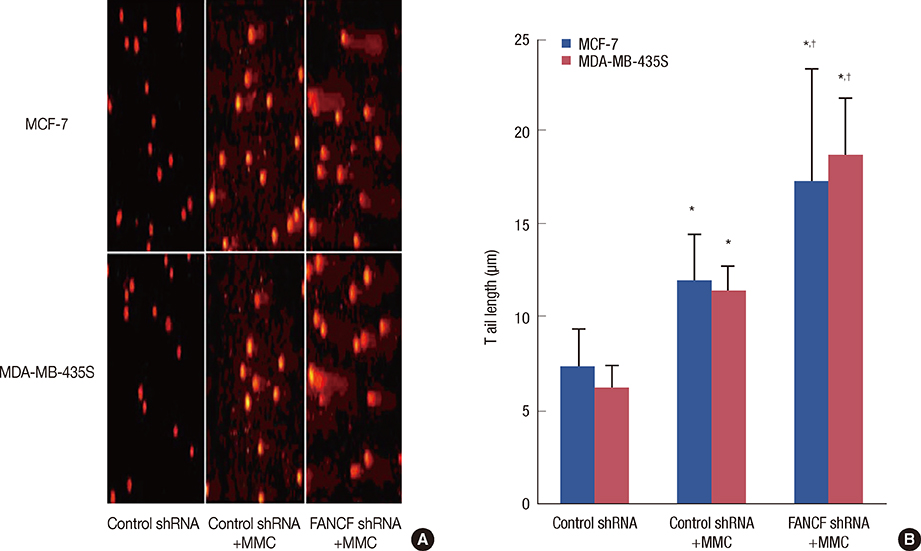
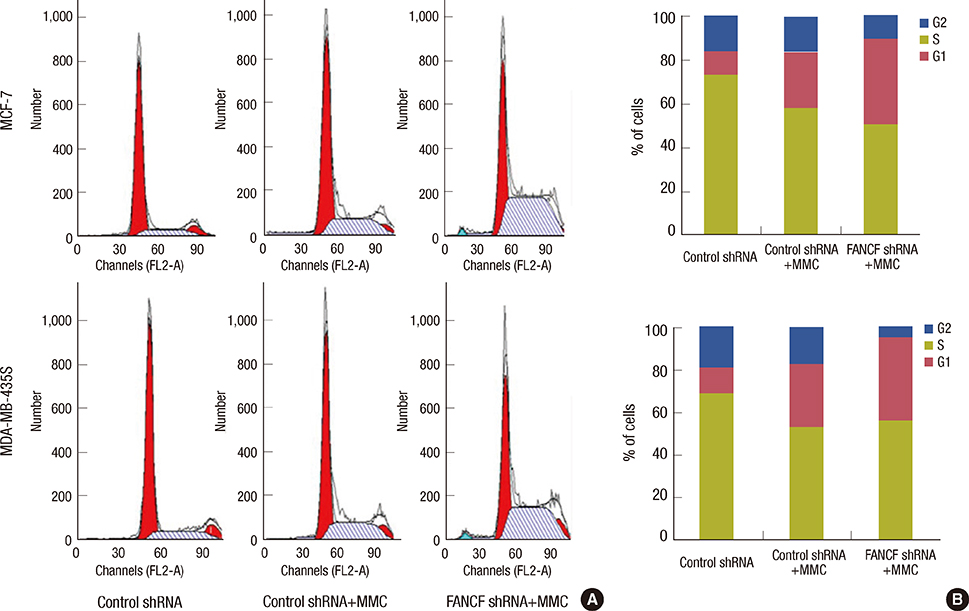
The analyses of two breast cancer cell lines (MCF-7 and MDA-MB-435S) demonstrated that the FANCF shRNA could effectively block the FA/BRCA pathway through the inhibition of Fanconi anemia complementation group D2 ubiquitination. Moreover, FANCF silencing potentiated the sensitivity of cells to mitomycin C (MMC), where combined FANCF shRNA/MMC treatment inhibited cell proliferation, induced S-phase arrest, apoptosis, and DNA fragmentation, and reduced the mitochondrial membrane potential, compared with MMC treatment alone.
CONCLUSION
Taken together, this study demonstrates that the inhibition of FANCF by its shRNA leads to a synergistic enhancement of MMC cytotoxicity in breast cancer cells. These results suggest that the inhibition of the FA/BRCA pathway is a useful adjunct to cytotoxic chemotherapy for the treatment of breast cancer.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Breast
Breast Neoplasms
Cell Count
Cell Cycle
Cell Line
Cell Line, Tumor
Cell Proliferation
Cell Survival
Comet Assay
Complement System Proteins
DNA Damage
DNA Fragmentation
Fanconi Anemia
Fanconi Anemia Complementation Group F Protein
Membrane Potential, Mitochondrial
Mitomycin
RNA, Small Interfering
Ubiquitin
Ubiquitination
Complement System Proteins
Fanconi Anemia Complementation Group F Protein
Mitomycin
RNA, Small Interfering
Ubiquitin
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.
Article2. Aldabbagh K, Pouderoux S, Roca L, Poujol S, Fabbro M, Romieu G, et al. Etoposide, mitomycin, and methotrexate combination in heavily treated breast cancer: a retrospective study. Breast Cancer. 2012; 19:16–22.
Article3. Urruticoechea A, Archer CD, Assersohn LA, Gregory RK, Verrill M, Mendes R, et al. Mitomycin C, vinblastine and cisplatin (MVP): an active and well-tolerated salvage regimen for advanced breast cancer. Br J Cancer. 2005; 92:475–479.
Article4. Celli CM, Jaiswal AK. Role of GRP58 in mitomycin C-induced DNA cross-linking. Cancer Res. 2003; 63:6016–6025.5. McHugh PJ, Spanswick VJ, Hartley JA. Repair of DNA interstrand crosslinks: molecular mechanisms and clinical relevance. Lancet Oncol. 2001; 2:483–490.
Article6. Bagby GC, Alter BP. Fanconi anemia. Semin Hematol. 2006; 43:147–156.
Article7. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009; 668:4–10.
Article8. Stoepker C, Hain K, Schuster B, Hilhorst-Hofstee Y, Rooimans MA, Steltenpool J, et al. SLX4, a coordinator of structure-specific endonucleases, is mutated in a new Fanconi anemia subtype. Nat Genet. 2011; 43:138–141.
Article9. Bogliolo M, Lyakhovich A, Callén E, Castellà M, Cappelli E, Ramírez MJ, et al. Histone H2AX and Fanconi anemia FANCD2 function in the same pathway to maintain chromosome stability. EMBO J. 2007; 26:1340–1351.
Article10. Lyakhovich A, Surrallés J. New roads to FA/BRCA pathway: H2AX. Cell Cycle. 2007; 6:1019–1023.
Article11. Olopade OI, Wei M. FANCF methylation contributes to chemoselectivity in ovarian cancer. Cancer Cell. 2003; 3:417–420.
Article12. Chen Q, Van der Sluis PC, Boulware D, Hazlehurst LA, Dalton WS. The FA/BRCA pathway is involved in melphalan-induced DNA interstrand cross-link repair and accounts for melphalan resistance in multiple myeloma cells. Blood. 2005; 106:698–705.
Article13. Narayan G, Arias-Pulido H, Nandula SV, Basso K, Sugirtharaj DD, Vargas H, et al. Promoter hypermethylation of FANCF: disruption of Fanconi Anemia-BRCA pathway in cervical cancer. Cancer Res. 2004; 64:2994–2997.14. Chen CC, Taniguchi T, D'Andrea A. The Fanconi anemia (FA) pathway confers glioma resistance to DNA alkylating agents. J Mol Med (Berl). 2007; 85:497–509.
Article15. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175:184–191.
Article16. Léveillé F, Blom E, Medhurst AL, Bier P, Laghmani el H, Johnson M, et al. The Fanconi anemia gene product FANCF is a flexible adaptor protein. J Biol Chem. 2004; 279:39421–39430.
Article17. Kowal P, Gurtan AM, Stuckert P, D'Andrea AD, Ellenberger T. Structural determinants of human FANCF protein that function in the assembly of a DNA damage signaling complex. J Biol Chem. 2007; 282:2047–2055.
Article18. Siegel D, Beall H, Senekowitsch C, Kasai M, Arai H, Gibson NW, et al. Bioreductive activation of mitomycin C by DT-diaphorase. Biochemistry. 1992; 31:7879–7885.
Article19. Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010; 29:405–434.
Article20. Tamm I, Schriever F, Dörken B. Apoptosis: implications of basic research for clinical oncology. Lancet Oncol. 2001; 2:33–42.
Article21. D'Andrea AD. The Fanconi Anemia/BRCA signaling pathway: disruption in cisplatin-sensitive ovarian cancers. Cell Cycle. 2003; 2:290–292.22. Burkitt K, Ljungman M. Phenylbutyrate interferes with the Fanconi anemia and BRCA pathway and sensitizes head and neck cancer cells to cisplatin. Mol Cancer. 2008; 7:24.
Article23. Hortobagyi GN. Mitomycin-C in breast cancer. Semin Oncol. 1985; 12:65–70.24. Godfrey TE. Mitomycin C in advanced breast cancer: an update. Semin Oncol. 1988; 15:71–73.25. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674.
Article26. Jiang YY, Wang HJ, Wang J, Tashiro S, Onodera S, Ikejima T. The protective effect of silibinin against mitomycin C-induced intrinsic apoptosis in human melanoma A375-S2 cells. J Pharmacol Sci. 2009; 111:137–146.
Article27. Moldovan GL, D'Andrea AD. How the fanconi anemia pathway guards the genome. Annu Rev Genet. 2009; 43:223–249.
Article28. Barroso E, Pita G, Arias JI, Menendez P, Zamora P, Blanco M, et al. The Fanconi anemia family of genes and its correlation with breast cancer susceptibility and breast cancer features. Breast Cancer Res Treat. 2009; 118:655–660.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overexpression of the Fanconi Anemia A Gene in Hela and MCF10A Cells
- Inactivation of the NHEJ Activity of DNA-PKcs Prevents Fanconi Anemia Pre-Leukemic HSC Expansion
- Treatment of Tongue Cancer in Patient with Fanconi's Anemia
- A Case Report of Fanconi Anemia Diagnosed by Genetic Testing Followed by Prenatal Diagnosis
- Chromosome Breakage Test for the Diagnosis of Fanconi's Anemia