Korean J Physiol Pharmacol.
2014 Dec;18(6):525-530. 10.4196/kjpp.2014.18.6.525.
TRPV1 in Salivary Gland Epithelial Cells Is Not Involved in Salivary Secretion via Transcellular Pathway
- Affiliations
-
- 1Department of Physiology, School of Dentistry, Seoul National University and Dental Research Institute, Seoul 110-749, Korea. kppark@snu.ac.kr
- 2Department of Physiology and Pathophysiology, Peking University Health Science Center, Beijing 100191, China.
- 3Department of Oral and Maxillofacial Surgery, Peking University School and Hospital of Stomatology, Beijing 100081, China.
- KMID: 2285556
- DOI: http://doi.org/10.4196/kjpp.2014.18.6.525
Abstract
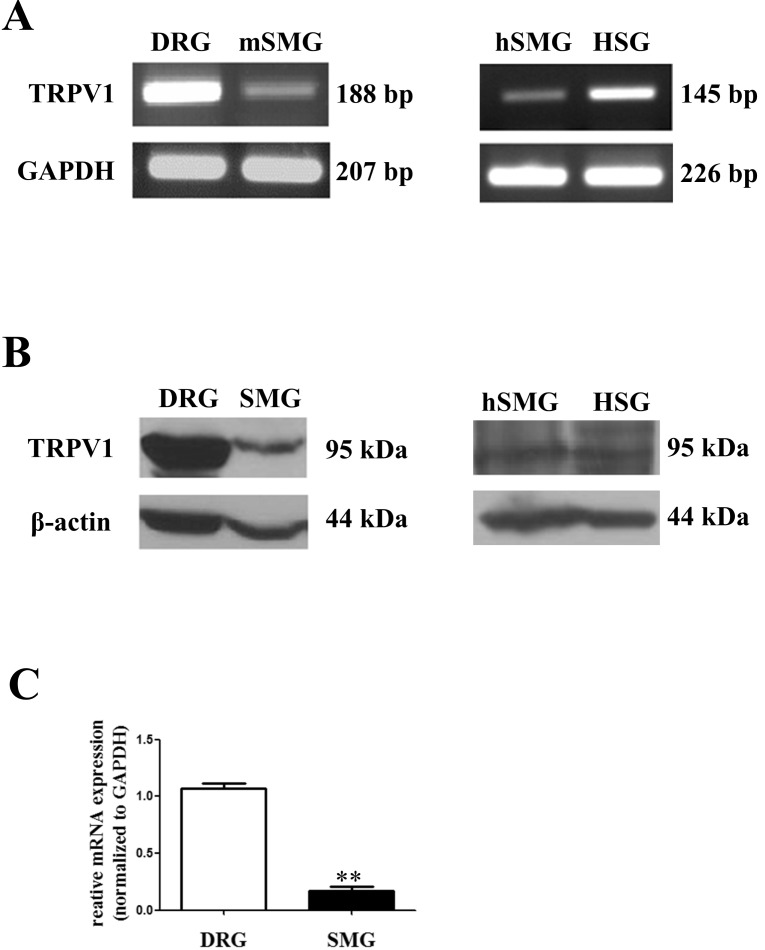
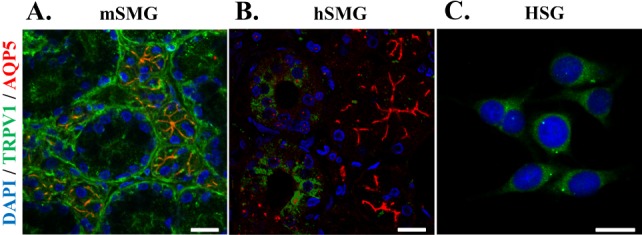
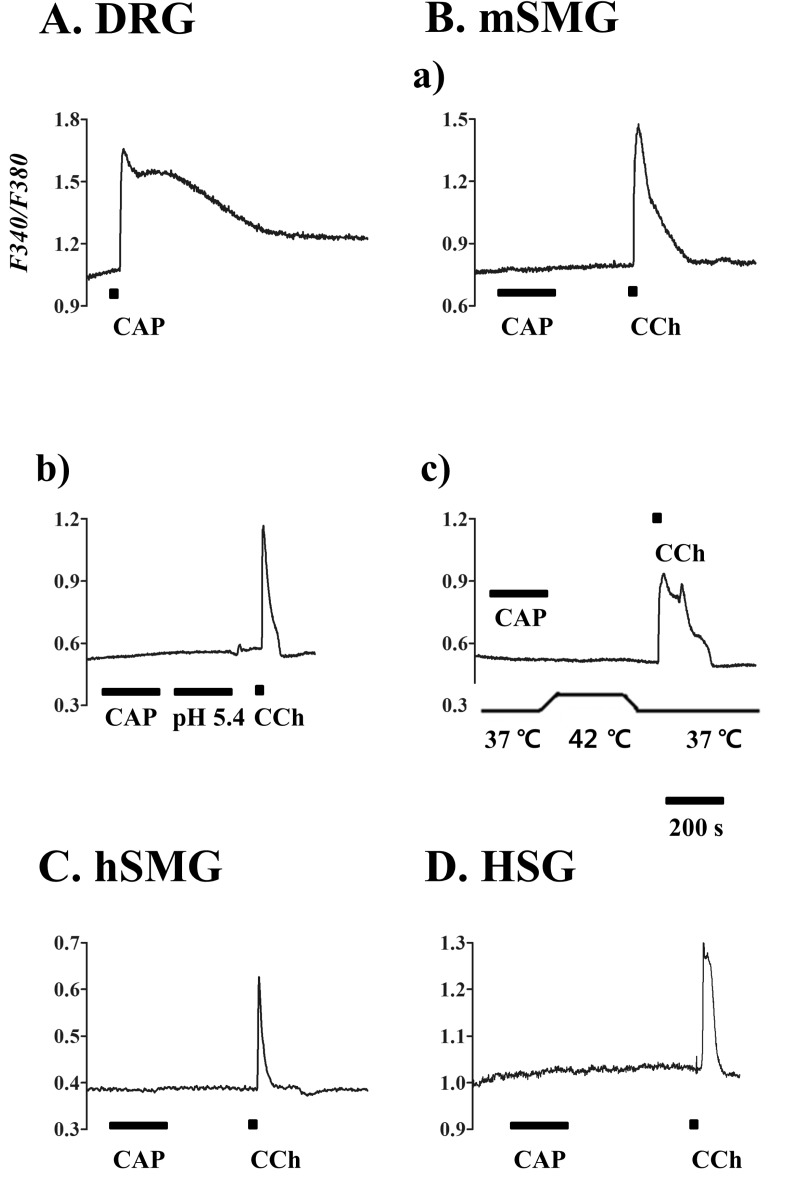
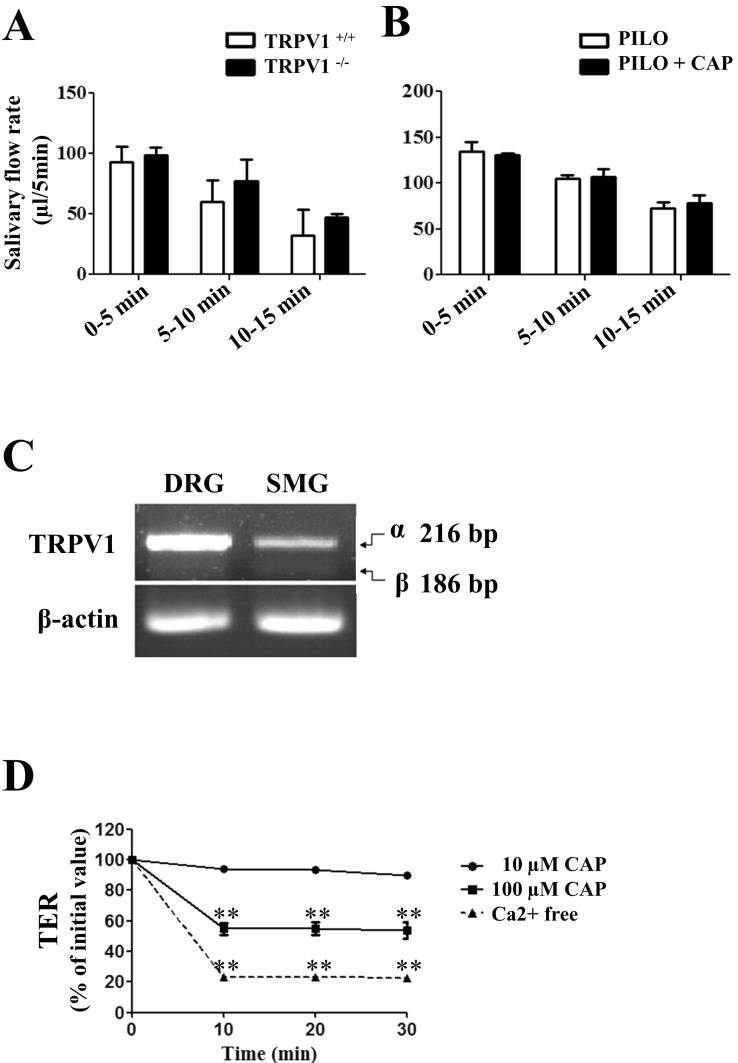
- Transient receptor potential vanilloid subtype 1 (TRPV1) was originally found in sensory neurons. Recently, it has been reported that TRPV1 is expressed in salivary gland epithelial cells (SGEC). However, the physiological role of TRPV1 in salivary secretion remains to be elucidated. We found that TRPV1 is expressed in mouse and human submandibular glands (SMG) and HSG cells, originated from human submandibular gland ducts at both mRNA and protein levels. However, capsaicin (CAP), TRPV1 agonist, had little effect on intracellular free calcium concentration ([Ca2+]i) in these cells, although carbachol consistently increased [Ca2+]i. Exposure of cells to high temperature (>43degrees C) or acidic bath solution (pH5.4) did not increase [Ca2+]i, either. We further examined the role of TRPV1 in salivary secretion using TRPV1 knock-out mice. There was no significant difference in the pilocarpine (PILO)-induced salivary flow rate between wild-type and TRPV1 knock-out mice. Saliva flow rate also showed insignificant change in the mice treated with PILO plus CAP compared with that in mice treated with PILO alone. Taken together, our results suggest that although TRPV1 is expressed in SGEC, it appears not to play any direct roles in saliva secretion via transcellular pathway.
MeSH Terms
Figure
Reference
-
1. Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002; 8:3–11. PMID: 11936453.
Article2. Ambudkar IS. Regulation of calcium in salivary gland secretion. Crit Rev Oral Biol Med. 2000; 11:4–25. PMID: 10682899.
Article3. Melvin JE, Yule D, Shuttleworth T, Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005; 67:445–469. PMID: 15709965.
Article4. Park K, Case RM, Brown PD. Identification and regulation of K+ and Cl- channels in human parotid acinar cells. Arch Oral Biol. 2001; 46:801–810. PMID: 11420052.5. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–824. PMID: 9349813.
Article6. Tominaga M, Caterina MJ. Thermosensation and pain. J Neurobiol. 2004; 61:3–12. PMID: 15362149.
Article7. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999; 51:159–212. PMID: 10353985.9. Veronesi B, Carter JD, Devlin RB, Simon SA, Oortgiesen M. Neuropeptides and capsaicin stimulate the release of inflammatory cytokines in a human bronchial epithelial cell line. Neuropeptides. 1999; 33:447–456. PMID: 10657523.
Article10. Inoue K, Koizumi S, Fuziwara S, Denda S, Inoue K, Denda M. Functional vanilloid receptors in cultured normal human epidermal keratinocytes. Biochem Biophys Res Commun. 2002; 291:124–129. PMID: 11829471.
Article11. Engler A, Aeschlimann A, Simmen BR, Michel BA, Gay RE, Gay S, Sprott H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem Biophys Res Commun. 2007; 359:884–888. PMID: 17560936.
Article12. Zhang Y, Xiang B, Li YM, Wang Y, Wang X, Wang YN, Wu LL, Yu GY. Expression and characteristics of vanilloid receptor 1 in the rabbit submandibular gland. Biochem Biophys Res Commun. 2006; 345:467–473. PMID: 16684507.
Article13. Ding QW, Zhang Y, Wang Y, Wang YN, Zhang L, Ding C, Wu LL, Yu GY. Functional vanilloid receptor-1 in human submandibular glands. J Dent Res. 2010; 89:711–716. PMID: 20371865.
Article14. Cong X, Zhang Y, Shi L, Yang NY, Ding C, Li J, Ding QW, Su YC, Xiang RL, Wu LL, Yu GY. Activation of transient receptor potential vanilloid subtype 1 increases expression and permeability of tight junction in normal and hyposecretory submandibular gland. Lab Invest. 2012; 92:753–768. PMID: 22391958.
Article15. Cong X, Zhang Y, Yang NY, Li J, Ding C, Ding QW, Su YC, Mei M, Guo XH, Wu LL, Yu GY. Occludin is required for TRPV1-modulated paracellular permeability in the submandibular gland. J Cell Sci. 2013; 126:1109–1121. PMID: 23345400.
Article16. Shin YH, Namkoong E, Choi S, Bae JS, Jin M, Hwang SM, Arote R, Choi SY, Park K. Capsaicin regulates the NF-κB pathway in salivary gland inflammation. J Dent Res. 2013; 92:547–552. PMID: 23603336.
Article17. Jin M, Hwang SM, Davies AJ, Shin Y, Bae JS, Lee JH, Lee EB, Song YW, Park K. Autoantibodies in primary Sjogren's syndrome patients induce internalization of muscarinic type 3 receptors. Biochim Biophys Acta. 2012; 1822:161–167. PMID: 22137887.18. Bae JS, Koo NY, Namkoong E, Davies AJ, Choi SK, Shin Y, Jin M, Hwang SM, Mikoshiba K, Park K. Chaperone stress 70 protein (STCH) binds and regulates two acid/base transporters NBCe1-B and NHE1. J Biol Chem. 2013; 288:6295–6305. PMID: 23303189.19. Ma T, Song Y, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J Biol Chem. 1999; 274:20071–20074. PMID: 10400615.
Article20. Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997; 273:C1378–C1385. PMID: 9357784.21. Wang C, Hu HZ, Colton CK, Wood JD, Zhu MX. An alternative splicing product of the murine trpv1 gene dominant negatively modulates the activity of TRPV1 channels. J Biol Chem. 2004; 279:37423–37430. PMID: 15234965.
Article22. Kawedia JD, Jiang M, Kulkarni A, Waechter HE, Matlin KS, Pauletti GM, Menon AG. The protein kinase A pathway contributes to Hg2+-induced alterations in phosphorylation and subcellular distribution of occludin associated with increased tight junction permeability of salivary epithelial cell monolayers. J Pharmacol Exp Ther. 2008; 326:829–837. PMID: 18550693.23. Jin Y, Kim J, Kwak J. Activation of the cGMP/Protein Kinase G Pathway by Nitric Oxide Can Decrease TRPV1 Activity in Cultured Rat Dorsal Root Ganglion Neurons. Korean J Physiol Pharmacol. 2012; 16:211–217. PMID: 22802704.
Article24. McGuirk SM, Dolphin AC. G-protein mediation in nociceptive signal transduction: an investigation into the excitatory action of bradykinin in a subpopulation of cultured rat sensory neurons. Neuroscience. 1992; 49:117–128. PMID: 1407541.
Article25. Scholz J, Woolf CJ. Can we conquer pain. Nat Neurosci. 2002; 5(Suppl):1062–1067. PMID: 12403987.
Article26. Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001; 411:957–962. PMID: 11418861.
Article27. Joe B, Rao UJ, Lokesh BR. Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol Cell Biochem. 1997; 169:125–134. PMID: 9089639.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple sialolithiasis in sublingual gland: report of a case
- Salivary Duct Carcinoma: 2 Case Reports
- Role of Homeostatic Changes in Salivary Gland Acinar Cells in Primary Sjöögren's Syndrome: A Review
- Effect of glucagon-like peptide 1 on salivary gland hypofunction in diabetic db/db mice
- Clinical and Histopathological Studies on Salivary Gland Epithelial Tumors