Korean J Physiol Pharmacol.
2014 Feb;18(1):61-66. 10.4196/kjpp.2014.18.1.61.
Reversal of Cisplatin Resistance by Epigallocatechin Gallate Is Mediated by Downregulation of Axl and Tyro 3 Expression in Human Lung Cancer Cells
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Catholic University of Daegu, Daegu 705-718, Korea.
- 2Department of Biochemistry and Molecular Biology, School of Medicine, Yeungnam University, Daegu 705-717, Korea. chlee2@ynu.ac.kr
- KMID: 2285492
- DOI: http://doi.org/10.4196/kjpp.2014.18.1.61
Abstract
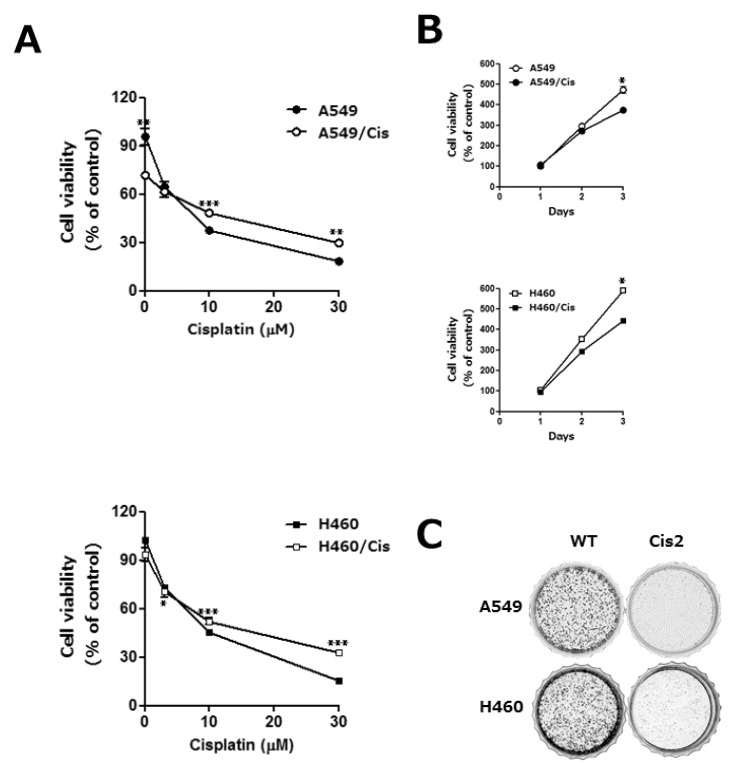
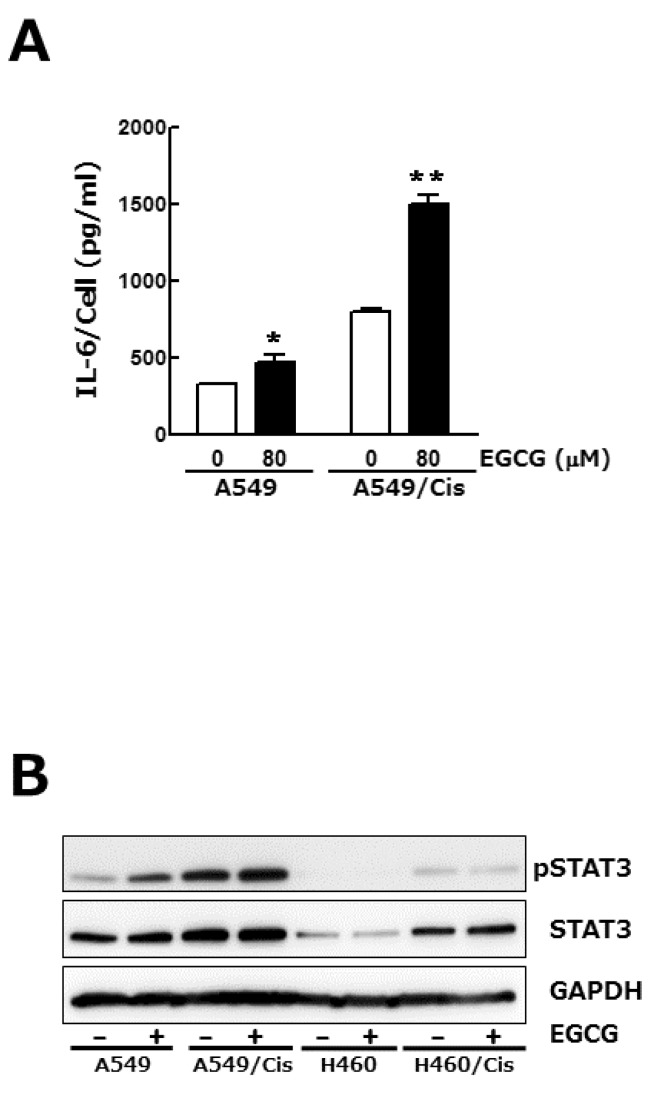
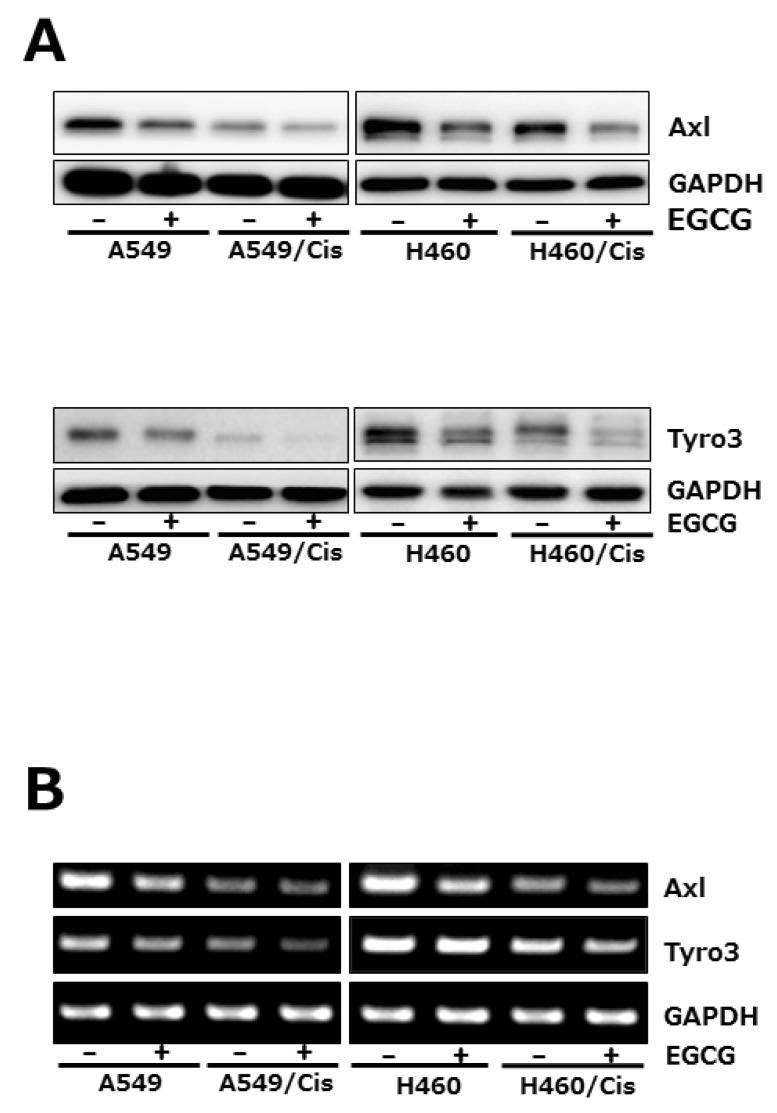
- Lung cancer is still the number one cause of death from cancer worldwide. The clinical effect of platinum-based chemotherapy for non-small cell lung cancer is constrained by the resistance to drug. To overcome chemo-resistance, various modified treatment including combination therapy has been used, but overall survival has not been improved yet. In this study, chemo-resistant lung cancer cells, A549/Cis and H460/Cis, were developed by long-term exposure of cells to cisplatin and the proliferative capability of these resistant cells was verified to be reduced. We found cytotoxic effect of epigallocatechin gallate (EGCG), a major catechin derived from green tea, on both the parental lung cancer cells, A549 and H460, and their cisplatin resistant cells, A549/Cis and H460/Cis. ELISA and Western blot analysis revealed that EGCG was able to increase interlukine-6 (IL-6) production per cell, whereas its downstream effector Signal transducers and activators of transcription 3 (STAT3) phosphorylation was not changed by EGCG, indicating that IL-6/STAT3 axis is not the critical signaling to be inhibited by EGCG. We next found that EGCG suppresses the expression of both Axl and Tyro 3 receptor tyrosine kinases at mRNA and protein level, explaining the cytotoxic effect of EGCG on lung cancer cells, especially, regardless of cisplatin resistance. Taken together, these data suggest that EGCG impedes proliferation of lung cancer cells including their chemo-resistant variants through downregulation of Axl and Tyro 3 expression.
Keyword
MeSH Terms
-
Axis, Cervical Vertebra
Blotting, Western
Carcinoma, Non-Small-Cell Lung
Catechin
Cause of Death
Cisplatin*
Down-Regulation*
Drug Therapy
Enzyme-Linked Immunosorbent Assay
Humans*
Lung Neoplasms*
Lung*
Parents
Phosphorylation
Phosphotransferases
RNA, Messenger
Tea
Transducers
Tyrosine
Catechin
Cisplatin
Phosphotransferases
RNA, Messenger
Tea
Tyrosine
Figure
Cited by 3 articles
-
Preventive Effect of Polysaccharide of
Larimichthys crocea Swim Bladder on Reserpine Induced Gastric Ulcer in ICR Mice
Gui-Jie Li, Peng Sun, Rui Wang, Ya-Lin Zhou, Yu Qian, Xin Zhao
Korean J Physiol Pharmacol. 2014;18(2):183-190. doi: 10.4196/kjpp.2014.18.2.183.MicroRNA-1 in Cardiac Diseases and Cancers
Jianzhe Li, Xiaomin Dong, Zhongping Wang, Jianhua Wu
Korean J Physiol Pharmacol. 2014;18(5):359-363. doi: 10.4196/kjpp.2014.18.5.359.Epigallocatechin-3-Gallate (EGCG) Attenuates Traumatic Brain Injury by Inhibition of Edema Formation and Oxidative Stress
Bo Zhang, Bing Wang, Shuhua Cao, Yongqiang Wang
Korean J Physiol Pharmacol. 2015;19(6):491-497. doi: 10.4196/kjpp.2015.19.6.491.
Reference
-
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29. PMID: 22237781.
Article2. Ferreira CG, Huisman C, Giaccone G. Novel approaches to the treatment of non-small cell lung cancer. Crit Rev Oncol Hematol. 2002; 41:57–77. PMID: 11796232.
Article3. Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000; 19:5548–5557. PMID: 11114734.
Article4. Sasaki T, Knyazev PG, Clout NJ, Cheburkin Y, Göhring W, Ullrich A, Timpl R, Hohenester E. Structural basis for Gas6-Axl signalling. EMBO J. 2006; 25:80–87. PMID: 16362042.
Article5. Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995; 80:661–670. PMID: 7867073.
Article6. Hong CC, Lay JD, Huang JS, Cheng AL, Tang JL, Lin MT, Lai GM, Chuang SE. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008; 268:314–324. PMID: 18502572.
Article7. Liu L, Greger J, Shi H, Liu Y, Greshock J, Annan R, Halsey W, Sathe GM, Martin AM, Gilmer TM. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: activation of AXL. Cancer Res. 2009; 69:6871–6878. PMID: 19671800.
Article8. Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, Kallop D, Ludlam MJ, Pei L. Axl as a potential therapeutic target in cancer: role of Axl in tumor growth, metastasis and angiogenesis. Oncogene. 2009; 28:3442–3455. PMID: 19633687.
Article9. Dunne PD, McArt DG, Blayney JK, Kalimutho M, Greer S, Wang T, Srivastava S, Ong CW, Arthur K, Loughrey M, Redmond K, Longley DB, Salto-Tellez M, Johnston PG, Van Schaeybroeck S. AXL is a key regulator of inherent and chemotherapy-induced invasion and predicts a poor clinical outcome in early-stage colon cancer. Clin Cancer Res. 2013; [Epub ahead of print].
Article10. Shieh YS, Lai CY, Kao YR, Shiah SG, Chu YW, Lee HS, Wu CW. Expression of axl in lung adenocarcinoma and correlation with tumor progression. Neoplasia. 2005; 7:1058–1064. PMID: 16354588.
Article11. Quong RY, Bickford ST, Ing YL, Terman B, Herlyn M, Lassam NJ. Protein kinases in normal and transformed melanocytes. Melanoma Res. 1994; 4:313–319. PMID: 7858416.
Article12. Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J, Wei K, Kuo CJ, Longacre TA, Giaccia AJ. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010; 70:7570–7579. PMID: 20858715.
Article13. Sainaghi PP, Castello L, Bergamasco L, Galletti M, Bellosta P, Avanzi GC. Gas6 induces proliferation in prostate carcinoma cell lines expressing the Axl receptor. J Cell Physiol. 2005; 204:36–44. PMID: 15605394.
Article14. Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting Axl and Mer kinases in cancer. Mol Cancer Ther. 2011; 10:1763–1773. PMID: 21933973.
Article15. Al-Ajmi N, Al-Maghrebi M, Renno WM. (-)-Epigallocatechin-3-gallate modulates the differential expression of survivin splice variants and protects spermatogenesis during testicular torsion. Korean J Physiol Pharmacol. 2013; 17:259–265. PMID: 23946684.
Article16. Yoon SS, Ahn KS, Kim SH, Shim YM, Kim J. In vitro establishment of cis-diammine-dichloroplatinum(II) resistant lung cancer cell line and modulation of apoptotic gene expression as a mechanism of resistant phenotype. Lung Cancer. 2001; 33:221–228. PMID: 11551417.
Article17. Wang J, Wang H, Zhao L, Fan S, Yang Z, Gao F, Chen L, Xiao GG, Molnár J, Wang Q. Down-regulation of P-glycoprotein is associated with resistance to cisplatin and VP-16 in human lung cancer cell lines. Anticancer Res. 2010; 30:3593–3598. PMID: 20944142.18. Shiau YC, Tsai SC, Wang JJ, Ho YJ, Ho ST, Kao CH. To predict chemotherapy response using technetium-99m tetrofosmin and compare with p-glycoprotein and multidrug resistance related protein-1 expression in patients with untreated small cell lung cancer. Cancer Lett. 2001; 169:181–188. PMID: 11431107.
Article19. Farabegoli F, Barbi C, Lambertini E, Piva R. (-)-Epigallocatechin-3-gallate downregulates estrogen receptor alpha function in MCF-7 breast carcinoma cells. Cancer Detect Prev. 2007; 31:499–504. PMID: 18061364.
Article20. Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003; 63:8118–8121. PMID: 14678963.21. Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, Lee MJ, Liu B, Guan F, Yang Z, Yu A, Yang CS. Pro-oxidative activities and dose-response relationship of (-)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010; 31:902–910. PMID: 20159951.
Article22. Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002; 3:651–662. PMID: 12209125.
Article23. Chang KT, Tsai CM, Chiou YC, Chiu CH, Jeng KS, Huang CY. IL-6 induces neuroendocrine dedifferentiation and cell proliferation in non-small cell lung cancer cells. Am J Physiol Lung Cell Mol Physiol. 2005; 289:L446–L453. PMID: 15894558.
Article24. Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012; 62:220–241. PMID: 22700443.
Article25. Wu H, Xin Y, Xiao Y, Zhao J. Low-dose docetaxel combined with (-)-epigallocatechin-3-gallate inhibits angiogenesis and tumor growth in nude mice with gastric cancer xenografts. Cancer Biother Radiopharm. 2012; 27:204–209. PMID: 22283637.
Article26. Mazumder ME, Beale P, Chan C, Yu JQ, Huq F. Epigallocatechin gallate acts synergistically in combination with cisplatin and designed trans-palladiums in ovarian cancer cells. Anticancer Res. 2012; 32:4851–4860. PMID: 23155251.27. Yang XW, Wang XL, Cao LQ, Jiang XF, Peng HP, Lin SM, Xue P, Chen D. Green tea polyphenol epigallocatechin-3-gallate enhances 5-fluorouracil-induced cell growth inhibition of hepatocellular carcinoma cells. Hepatol Res. 2012; 42:494–501. PMID: 22221825.
Article28. Culig Z, Puhr M. Interleukin-6: a multifunctional targetable cytokine in human prostate cancer. Mol Cell Endocrinol. 2012; 360:52–58. PMID: 21664423.
Article29. O'Bryan JP, Frye RA, Cogswell PC, Neubauer A, Kitch B, Prokop C, Espinosa R 3rd, Le Beau MM, Earp HS, Liu ET. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell Biol. 1991; 11:5016–5031. PMID: 1656220.30. Neubauer A, O'Bryan JP, Fiebeler A, Schmidt C, Huhn D, Liu ET. Axl, a novel receptor tyrosine kinase isolated from chronic myelogenous leukemia. Semin Hematol. 1993; 30(3 Suppl 3):34. PMID: 8235704.31. Wimmel A, Glitz D, Kraus A, Roeder J, Schuermann M. Axl receptor tyrosine kinase expression in human lung cancer cell lines correlates with cellular adhesion. Eur J Cancer. 2001; 37:2264–2274. PMID: 11677117.
Article32. Linger RM, Keating AK, Earp HS, Graham DK. Taking aim at Mer and Axl receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert Opin Ther Targets. 2010; 14:1073–1090. PMID: 20809868.
Article33. Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, Weimer R, Wu Y, Pei L. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010; 29:5254–5264. PMID: 20603615.
Article34. Postel-Vinay S, Ashworth A. AXL and acquired resistance to EGFR inhibitors. Nat Genet. 2012; 44:835–836. PMID: 22836088.
Article35. Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK, Choi YJ, Choi CM, Kim SW, Jang SJ, Park YS, Kim WS, Lee DH, Lee JS, Miller VA, Arcila M, Ladanyi M, Moonsamy P, Sawyers C, Boggon TJ, Ma PC, Costa C, Taron M, Rosell R, Halmos B, Bivona TG. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012; 44:852–860. PMID: 22751098.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overcoming the Intrinsic Gefitinib-resistance via Downregulation of AXL in Non-small Cell Lung Cancer
- GBA inhibition suppresses ovarian cancer growth, survival and receptor tyrosine kinase AXL-mediated signaling pathways
- The relationship between cisplatin-induced apoptosis and p53, bcl-2 and bax expression in human lung cancer cells
- Immunotherapy with methyl gallate, an inhibitor of Treg cell migration, enhances the anti-cancer effect of cisplatin therapy
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts