Korean J Physiol Pharmacol.
2023 Jan;27(1):21-29. 10.4196/kjpp.2023.27.1.21.
GBA inhibition suppresses ovarian cancer growth, survival and receptor tyrosine kinase AXL-mediated signaling pathways
- Affiliations
-
- 1Department of Gynecology, Wuhan Third Hospital-Tongren Hospital of Wuhan University, Wuhan 430064, China
- 2Department of Obstetrics and Gynaecology, Taikang Tongji (Wuhan) Hospital, Wuhan 430050, China
- KMID: 2537500
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.21
Abstract
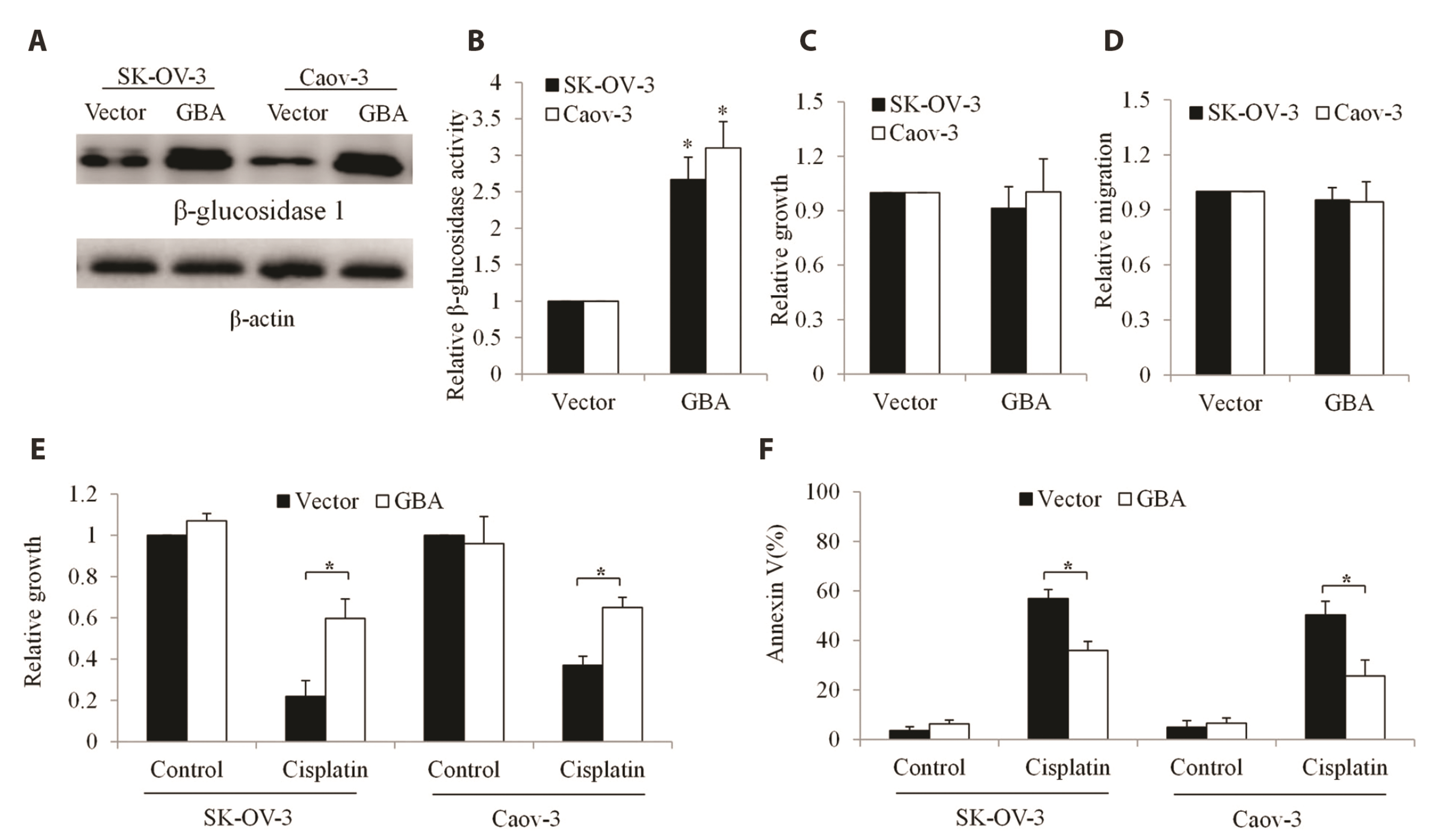
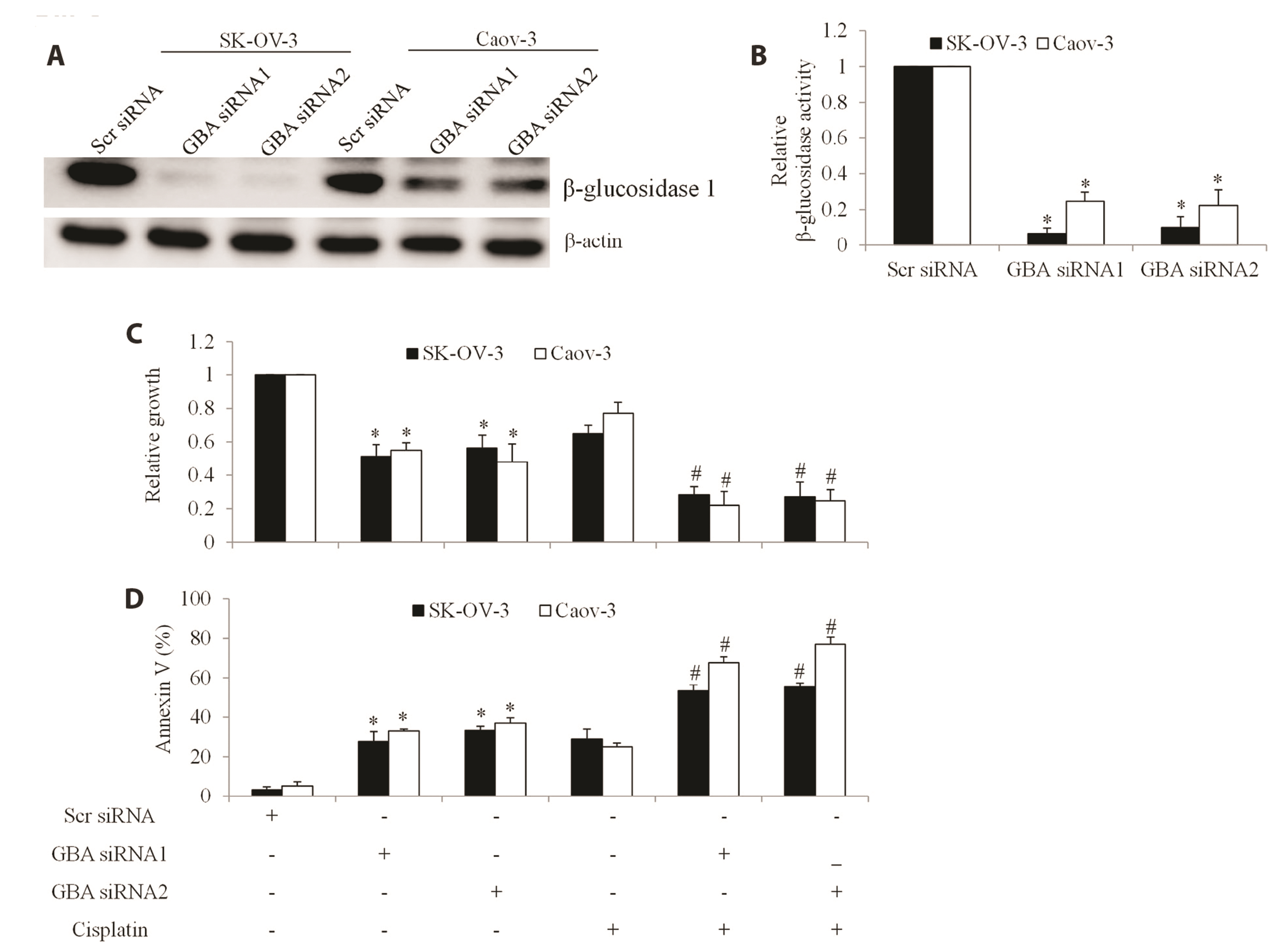
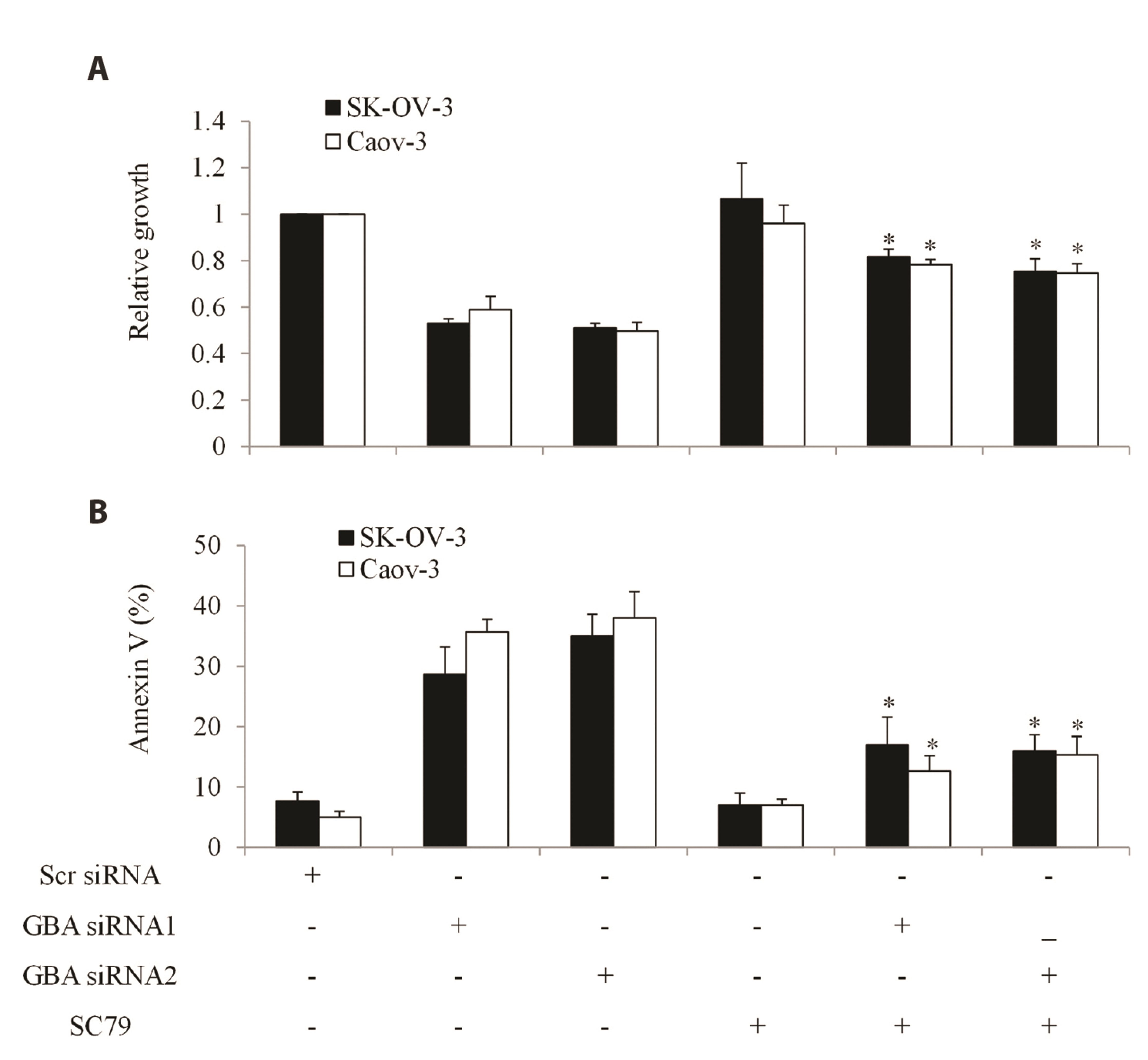
- The poor outcome of advanced ovarian cancer under conventional therapy necessitates new strategies to improve therapeutic efficacy. β-glucosidase (encoded by GBA) is a lysosomal enzyme and is involved in sphingolipids metabolism. Recent studies revealed that β-glucosidase plays a role in cancer development and chemoresistance. In this work, we systematically evaluated the expression and role of GBA in ovarian cancer. Our work demonstrates that inhibition of β-glucosidase has therapeutic potential for ovarian cancer. Gene Expression Profiling Interactive Analysis database, western blot and immunohistochemistry analyses of patient samples demonstrated that GBA mRNA and protein expression levels were significantly increased in ovarian cancer compared to normal tissues. Functional studies using gainof-function and loss-of-function approaches demonstrated that GBA overexpression did not affect growth and migration but alleviated cisplatin’s efficacy in ovarian cancer cells. In addition, GBA depletion resulted in growth inhibition, apoptosis induction, and enhancement of cisplatin’s efficacy. Of note, we found that GBA inhibition specifically decreased receptor tyrosine kinase AXL level, leading to the suppression of AXL-mediated signaling pathways. Our data suggest that GBA represents a promising target to inhibit AXL signaling and overcome cisplatin resistance in ovarian cancer.
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. Erratum in: CA Cancer J Clin. 2020;70:313. DOI: 10.3322/caac.21492. PMID: 30207593. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053395052&origin=inward.2. Raja FA, Chopra N, Ledermann JA. 2012; Optimal first-line treatment in ovarian cancer. Ann Oncol. 23 Suppl 10:x118–x127. DOI: 10.1093/annonc/mds315. PMID: 22987945. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84866749053&origin=inward.3. Grabowski JP, Sehouli J. 2015; Current management of ovarian cancer. Minerva Med. 106:151–156. PMID: 25900837. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84964525466&origin=inward.4. Agarwal R, Kaye SB. 2003; Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 3:502–516. DOI: 10.1038/nrc1123. PMID: 12835670. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0043240182&origin=inward.5. Norouzi-Barough L, Sarookhani MR, Sharifi M, Moghbelinejad S, Jangjoo S, Salehi R. 2018; Molecular mechanisms of drug resistance in ovarian cancer. J Cell Physiol. 233:4546–4562. DOI: 10.1002/jcp.26289. PMID: 29152737. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042552250&origin=inward.6. Grabowski GA, Osiecki-Newman K, Dinur T, Fabbro D, Legler G, Gatt S, Desnick RJ. 1986; Human acid beta-glucosidase. Use of conduritol B epoxide derivatives to investigate the catalytically active normal and Gaucher disease enzymes. J Biol Chem. 261:8263–8269. DOI: 10.1016/S0021-9258(19)83905-4. PMID: 3087971.7. Aureli M, Masilamani AP, Illuzzi G, Loberto N, Scandroglio F, Prinetti A, Chigorno V, Sonnino S. 2009; Activity of plasma membrane beta-galactosidase and beta-glucosidase. FEBS Lett. 583:2469–2473. DOI: 10.1016/j.febslet.2009.06.048. PMID: 19577566. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67849109791&origin=inward.8. Futerman AH, Sussman JL, Horowitz M, Silman I, Zimran A. 2004; New directions in the treatment of Gaucher disease. Trends Pharmacol Sci. 25:147–151. DOI: 10.1016/j.tips.2004.01.004. PMID: 15019270. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=1342306744&origin=inward.9. Li Y, Sekine T, Funayama M, Li L, Yoshino H, Nishioka K, Tomiyama H, Hattori N. 2014; Clinicogenetic study of GBA mutations in patients with familial Parkinson's disease. Neurobiol Aging. 35:935.e3–e8. DOI: 10.1016/j.neurobiolaging.2013.09.019. PMID: 24126159. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85058205837&origin=inward.10. Chahine LM, Qiang J, Ashbridge E, Minger J, Yearout D, Horn S, Colcher A, Hurtig HI, Lee VM, Van Deerlin VM, Leverenz JB, Siderowf AD, Trojanowski JQ, Zabetian CP, Chen-Plotkin A. 2013; Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol. 70:852–858. DOI: 10.1001/jamaneurol.2013.1274. PMID: 23699752. PMCID: PMC3762458. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880296731&origin=inward.11. Futerman AH, van Meer G. 2004; The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 5:554–565. DOI: 10.1038/nrm1423. PMID: 15232573. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2942687937&origin=inward.12. Sun Y, Zhang W, Xu YH, Quinn B, Dasgupta N, Liou B, Setchell KD, Grabowski GA. 2013; Substrate compositional variation with tissue/region and Gba1 mutations in mouse models--implications for Gaucher disease. PLoS One. 8:e57560. DOI: 10.1371/journal.pone.0057560. PMID: 23520473. PMCID: PMC3592923. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84874761253&origin=inward.13. Kitatani K, Sheldon K, Anelli V, Jenkins RW, Sun Y, Grabowski GA, Obeid LM, Hannun YA. 2009; Acid beta-glucosidase 1 counteracts p38delta-dependent induction of interleukin-6: possible role for ceramide as an anti-inflammatory lipid. J Biol Chem. 284:12979–12988. DOI: 10.1074/jbc.M809500200. PMID: 19279008. PMCID: PMC2676030. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67649752327&origin=inward.14. Arafa HM. 2009; Possible contribution of beta-glucosidase and caspases in the cytotoxicity of glufosfamide in colon cancer cells. Eur J Pharmacol. 616:58–63. DOI: 10.1016/j.ejphar.2009.06.024. PMID: 19545561. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67651108804&origin=inward.15. Hirano T. 2021; IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 33:127–148. DOI: 10.1093/intimm/dxaa078. PMID: 33337480. PMCID: PMC7799025. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85102658220&origin=inward.16. Zhang Y, Zhu K, Miao X, Hu X, Wang T. 2016; Identification of β-glucosidase 1 as a biomarker and its high expression in hepatocellular carcinoma is associated with resistance to chemotherapy drugs. Biomarkers. 21:249–256. DOI: 10.3109/1354750X.2015.1134662. PMID: 26849828. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84958049578&origin=inward.17. Zhou X, Huang Z, Yang H, Jiang Y, Wei W, Li Q, Mo Q, Liu J. 2017; β-Glucosidase inhibition sensitizes breast cancer to chemotherapy. Biomed Pharmacother. 91:504–509. DOI: 10.1016/j.biopha.2017.04.113. PMID: 28478274. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85018776558&origin=inward.18. Li Z, Xu D, Tong X, Shan C. 2021; Inhibition of β-glucosidase overcomes gastric cancer chemoresistance through inducing lysosomal dysfunction. Clin Res Hepatol Gastroenterol. 45:101456. DOI: 10.1016/j.clinre.2020.04.020. PMID: 32507687. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085930739&origin=inward.19. Shi SR, Liu C, Young L, Taylor C. 2007; Development of an optimal antigen retrieval protocol for immunohistochemistry of retinoblastoma protein (pRB) in formalin fixed, paraffin sections based on comparison of different methods. Biotech Histochem. 82:301–309. DOI: 10.1080/10520290701791763. PMID: 18097796. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=38449121657&origin=inward.20. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. 2017; GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102. DOI: 10.1093/nar/gkx247. PMID: 28407145. PMCID: PMC5570223. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85023171204&origin=inward.21. Hernandez L, Kim MK, Lyle LT, Bunch KP, House CD, Ning F, Noonan AM, Annunziata CM. 2016; Characterization of ovarian cancer cell lines as in vivo models for preclinical studies. Gynecol Oncol. 142:332–340. DOI: 10.1016/j.ygyno.2016.05.028. PMID: 27235858. PMCID: PMC4961516. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84970028733&origin=inward.22. Vathipadiekal V, Saxena D, Mok SC, Hauschka PV, Ozbun L, Birrer MJ. 2012; Identification of a potential ovarian cancer stem cell gene expression profile from advanced stage papillary serous ovarian cancer. PLoS One. 7:e29079. DOI: 10.1371/journal.pone.0029079. PMID: 22272227. PMCID: PMC3260150. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84855857047&origin=inward.23. Elkabets M, Pazarentzos E, Juric D, Sheng Q, Pelossof RA, Brook S, Benzaken AO, Rodon J, Morse N, Yan JJ, Liu M, Das R, Chen Y, Tam A, Wang H, Liang J, Gurski JM, Kerr DA, Rosell R, Teixidó C, et al. 2015; AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 27:533–546. DOI: 10.1016/j.ccell.2015.03.010. PMID: 25873175. PMCID: PMC4398915. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84928015512&origin=inward.24. Jo H, Mondal S, Tan D, Nagata E, Takizawa S, Sharma AK, Hou Q, Shanmugasundaram K, Prasad A, Tung JK, Tejeda AO, Man H, Rigby AC, Luo HR. 2012; Small molecule-induced cytosolic activation of protein kinase Akt rescues ischemia-elicited neuronal death. Proc Natl Acad Sci U S A. 109:10581–10586. DOI: 10.1073/pnas.1202810109. PMID: 22689977. PMCID: PMC3387065. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84862998910&origin=inward.25. Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S. 2021; Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol. 13:303–328. DOI: 10.2147/JEP.S267383. PMID: 33776489. PMCID: PMC7987268. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85103132176&origin=inward.26. Kitatani K, Sheldon K, Rajagopalan V, Anelli V, Jenkins RW, Sun Y, Grabowski GA, Obeid LM, Hannun YA. 2009; Involvement of acid beta-glucosidase 1 in the salvage pathway of ceramide formation. J Biol Chem. 284:12972–12978. DOI: 10.1074/jbc.M802790200. PMID: 19279011. PMCID: PMC2676029. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=67649710875&origin=inward.27. Dinur T, Osiecki KM, Legler G, Gatt S, Desnick RJ, Grabowski GA. 1986; Human acid beta-glucosidase: isolation and amino acid sequence of a peptide containing the catalytic site. Proc Natl Acad Sci U S A. 83:1660–1664. DOI: 10.1073/pnas.83.6.1660. PMID: 3456607. PMCID: PMC323143. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0343646886&origin=inward.28. Ogretmen B. 2018; Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 18:33–50. DOI: 10.1038/nrc.2017.96. PMID: 29147025. PMCID: PMC5818153. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85038830855&origin=inward.29. Gay CM, Balaji K, Byers LA. 2017; Giving AXL the axe: targeting AXL in human malignancy. Br J Cancer. 116:415–423. DOI: 10.1038/bjc.2016.428. PMID: 28072762. PMCID: PMC5318970. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85009188501&origin=inward.30. Zhu C, Wei Y, Wei X. 2019; AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 18:153. DOI: 10.1186/s12943-019-1090-3. PMID: 31684958. PMCID: PMC6827209. PMID: 34f90097190e412d9db5558bc7397754. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85074548394&origin=inward.31. Shorthouse D, Hedger G, Koldsø H, Sansom MS. 2016; Molecular simulations of glycolipids: towards mammalian cell membrane models. Biochimie. 120:105–109. DOI: 10.1016/j.biochi.2015.09.033. PMID: 26427555. PMCID: PMC4710579.32. Macleod K, Mullen P, Sewell J, Rabiasz G, Lawrie S, Miller E, Smyth JF, Langdon SP. 2005; Altered ErbB receptor signaling and gene expression in cisplatin-resistant ovarian cancer. Cancer Res. 65:6789–6800. DOI: 10.1158/0008-5472.CAN-04-2684. PMID: 16061661. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=23044504066&origin=inward.33. Quinn JM, Greenwade MM, Palisoul ML, Opara G, Massad K, Guo L, Zhao P, Beck-Noia H, Hagemann IS, Hagemann AR, McCourt CK, Thaker PH, Powell MA, Mutch DG, Fuh KC. 2019; Therapeutic inhibition of the receptor tyrosine kinase AXL improves sensitivity to platinum and taxane in ovarian cancer. Mol Cancer Ther. 18:389–398. DOI: 10.1158/1535-7163.MCT-18-0537. PMID: 30478151. PMCID: PMC6363844. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85061038639&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Overcoming the Intrinsic Gefitinib-resistance via Downregulation of AXL in Non-small Cell Lung Cancer
- Synergistic Induction of Apoptosis by the Combination of an Axl Inhibitor and Auranofin in Human Breast Cancer Cells
- Growth inhibition in head and neck cancer cell lines by gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor
- Molecular Basis of Drug Resistance: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors and Anaplastic Lymphoma Kinase Inhibitors
- Culture Conditions for Mycelial Growth and Anti-Cancer Properties of Termitomyces