Korean J Physiol Pharmacol.
2013 Aug;17(4):307-314. 10.4196/kjpp.2013.17.4.307.
High Glucose Induces Connective Tissue Growth Factor Expression and Extracellular Matrix Accumulation in Rat Aorta Vascular Smooth Muscle Cells Via Extracellular Signal-Regulated Kinase 1/2
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Yeungnam University, Daegu 705-717, Korea. yjkang@med.yu.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, College of Medicine, Yeungnam University, Daegu 705-717, Korea.
- 3Department of Aging-Associated Vascular Disease Research Center, College of Medicine, Yeungnam University, Daegu 705-717, Korea.
- KMID: 2285465
- DOI: http://doi.org/10.4196/kjpp.2013.17.4.307
Abstract
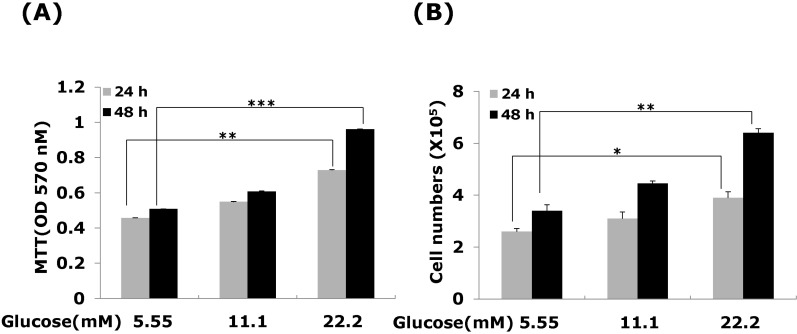
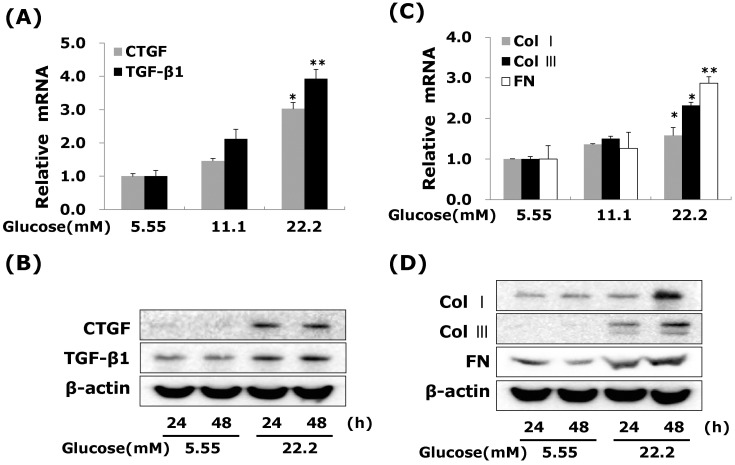
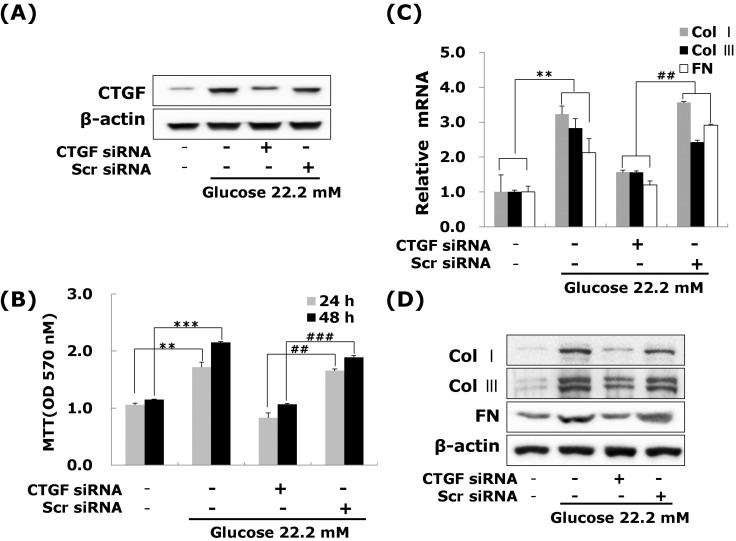
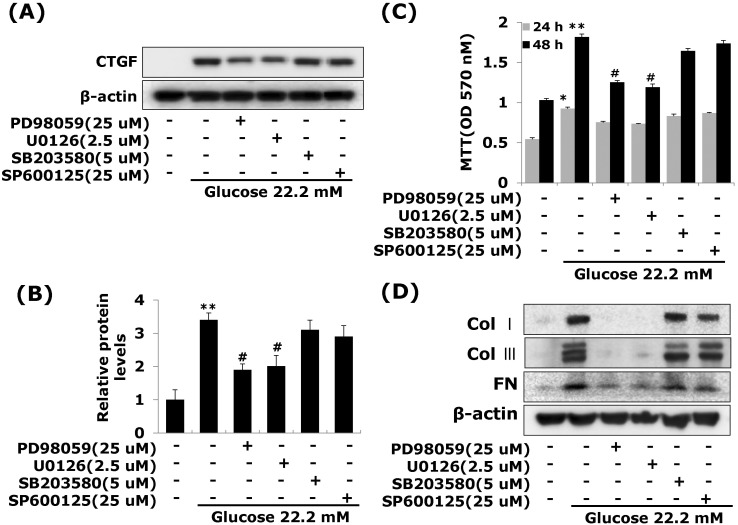
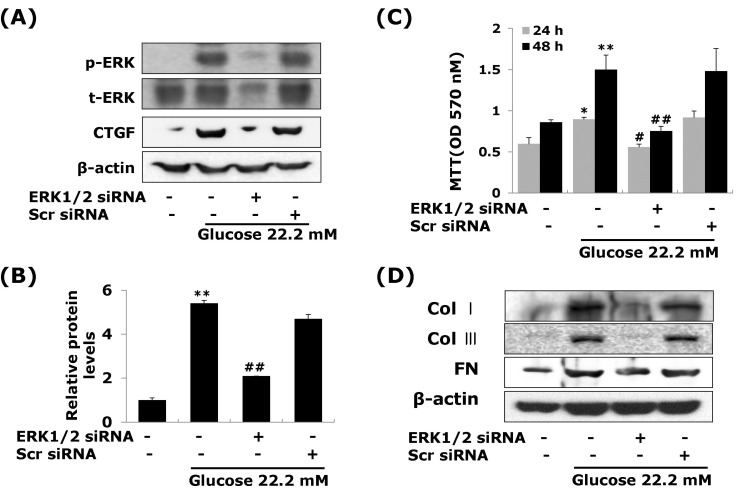
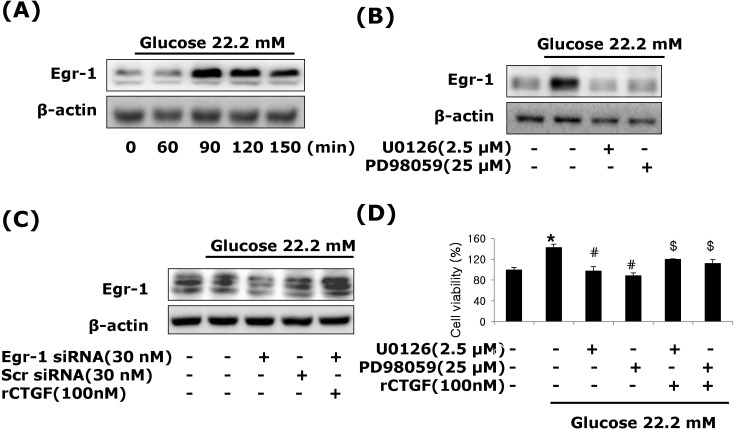
- Connective tissue growth factor (CTGF) is a potent pro-fibrotic factor, which is implicated in fibrosis through extracellular matrix (ECM) induction in diabetic cardiovascular complications. It is an important downstream mediator in the fibrotic action of transforming growth factor beta (TGFbeta) and is potentially induced by hyperglycemia in human vascular smooth muscle cells (VSMCs). Therefore, the goal of this study is to identify the signaling pathways of CTGF effects on ECM accumulation and cell proliferation in VSMCs under hyperglycemia. We found that high glucose stimulated the levels of CTGF mRNA and protein and followed by VSMC proliferation and ECM components accumulation such as collagen type 1, collagen type 3 and fibronectin. By depleting endogenous CTGF we showed that CTGF is indispensable for the cell proliferation and ECM components accumulation in high glucose-stimulated VSMCs. In addition, pretreatment with the MEK1/2 specific inhibitors, PD98059 or U0126 potently inhibited the CTGF production and ECM components accumulation in high glucose-stimulated VSMCs. Furthermore, knockdown with ERK1/2 MAPK siRNA resulted in significantly down regulated of CTGF production, ECM components accumulation and cell proliferation in high glucose-stimulated VSMCs. Finally, ERK1/2 signaling regulated Egr-1 protein expression and treatment with recombinant CTGF reversed the Egr-1 expression in high glucose-induced VSMCs. It is conceivable that ERK1/2 MAPK signaling pathway plays an important role in regulating CTGF expression and suggests that blockade of CTGF through ERK1/2 MAPK signaling may be beneficial for therapeutic target of diabetic cardiovascular complication such as atherosclerosis.
Keyword
MeSH Terms
-
Animals
Aorta
Atherosclerosis
Butadienes
Cell Proliferation
Collagen
Connective Tissue
Connective Tissue Growth Factor
Diabetes Complications
Extracellular Matrix
Fibronectins
Fibrosis
Flavonoids
Glucose
Humans
Hyperglycemia
Muscle, Smooth, Vascular
Nitriles
Phosphotransferases
Rats
RNA, Messenger
RNA, Small Interfering
Transforming Growth Factor beta
Butadienes
Collagen
Connective Tissue Growth Factor
Fibronectins
Flavonoids
Glucose
Nitriles
Phosphotransferases
RNA, Messenger
RNA, Small Interfering
Transforming Growth Factor beta
Figure
Cited by 2 articles
-
Fluvastatin inhibits advanced glycation end products-induced proliferation, migration, and extracellular matrix accumulation in vascular smooth muscle cells by targeting connective tissue growth factor
Ae-Rang Hwang, Ju-Ock Nam, Young Jin Kang
Korean J Physiol Pharmacol. 2018;22(2):193-201. doi: 10.4196/kjpp.2018.22.2.193.Long Term Effect of High Glucose and Phosphate Levels on the OPG/RANK/RANKL/TRAIL System in the Progression of Vascular Calcification in rat Aortic Smooth Muscle Cells
Yang Ho Kang, Jung Sook Jin, Seok Man Son
Korean J Physiol Pharmacol. 2015;19(2):111-118. doi: 10.4196/kjpp.2015.19.2.111.
Reference
-
1. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002; 287:2570–2581. PMID: 12020339.2. Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005; 54:1–7. PMID: 15616004.3. Aouichat Bouguerra S, Benazzoug Y, Bekkhoucha F, Bourdillon MC. Effect of high glucose concentration on collagen synthesis and cholesterol level in the phenotypic modulation of aortic cultured smooth muscle cells of sand rat (Psammomys obesus). Exp Diabesity Res. 2004; 5:227–235. PMID: 15512791.4. McGinn S, Poronnik P, Gallery ED, Pollock CA. The effects of high glucose and atorvastatin on endothelial cell matrix production. Diabet Med. 2004; 21:1102–1107. PMID: 15384957.
Article5. Yevdokimova NY, Komisarenko SV. TGFbeta1 is involved in high glucose-induced accumulation of pericellular chondroitin sulphate in human endothelial cells. J Diabetes Complications. 2004; 18:300–308. PMID: 15337504.
Article6. Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003; 63:2010–2019. PMID: 12753288.7. Kobayashi T, Inoue T, Okada H, Kikuta T, Kanno Y, Nishida T, Takigawa M, Sugaya T, Suzuki H. Connective tissue growth factor mediates the profibrotic effects of transforming growth factor-beta produced by tubular epithelial cells in response to high glucose. Clin Exp Nephrol. 2005; 9:114–121. PMID: 15980944.8. McLennan SV, Wang XY, Moreno V, Yue DK, Twigg SM. Connective tissue growth factor mediates high glucose effects on matrix degradation through tissue inhibitor of matrix metalloproteinase type 1: implications for diabetic nephropathy. Endocrinology. 2004; 145:5646–5655. PMID: 15345671.
Article9. Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008; 4:575–596. PMID: 18827908.10. Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007; 117:557–567. PMID: 17332883.
Article11. Mu Y, Gudey SK, Landstörm M. Non-Smad signaling pathways. Cell Tissue Res. 2012; 347:11–20. PMID: 21701805.
Article12. Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002; 82:85–91. PMID: 12008039.13. Karkampouna S, Ten Dijke P, Dooley S, Julio MK. TGFβ signaling in liver regeneration. Curr Pharm Des. 2012; 18:4103–4113. PMID: 22630085.
Article14. Oemar BS, Lüscher TF. Connective tissue growth factor. Friend or foe? Arterioscler Thromb Vasc Biol. 1997; 17:1483–1489. PMID: 9301624.15. Cicha I, Yilmaz A, Klein M, Raithel D, Brigstock DR, Daniel WG, Goppelt-Struebe M, Garlichs CD. Connective tissue growth factor is overexpressed in complicated atherosclerotic plaques and induces mononuclear cell chemotaxis in vitro. Arterioscler Thromb Vasc Biol. 2005; 25:1008–1013. PMID: 15761189.16. Kundi R, Hollenbeck ST, Yamanouchi D, Herman BC, Edlin R, Ryer EJ, Wang C, Tsai S, Liu B, Kent KC. Arterial gene transfer of the TGF-beta signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc Res. 2009; 84:326–335. PMID: 19570811.17. Li X, Liu W, Wang Q, Liu P, Deng Y, Lan T, Zhang X, Qiu B, Ning H, Huang H. Emodin suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Mol Cell Endocrinol. 2009; 307:157–162. PMID: 19524136.
Article18. Lam S, van der Geest RN, Verhagen NA, van Nieuwenhoven FA, Blom IE, Aten J, Goldschmeding R, Daha MR, van Kooten C. Connective tissue growth factor and igf-I are produced by human renal fibroblasts and cooperate in the induction of collagen production by high glucose. Diabetes. 2003; 52:2975–2983. PMID: 14633859.
Article19. Liu X, Luo F, Pan K, Wu W, Chen H. High glucose upregulates connective tissue growth factor expression in human vascular smooth muscle cells. BMC Cell Biol. 2007; 8:1. PMID: 17224075.
Article20. Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001; 20:755–766. PMID: 11179220.
Article21. Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003; 63:2010–2019. PMID: 12753288.22. Srivastava S, Ramana KV, Tammali R, Srivastava SK, Bhatnagar A. Contribution of aldose reductase to diabetic hyperproliferation of vascular smooth muscle cells. Diabetes. 2006; 55:901–910. PMID: 16567509.
Article23. Jiang Z, Yu P, Tao M, Fernandez C, Ifantides C, Moloye O, Schultz GS, Ozaki CK, Berceli SA. TGF-beta- and CTGF-mediated fibroblast recruitment influences early outward vein graft remodeling. Am J Physiol Heart Circ Physiol. 2007; 293:H482–H488. PMID: 17369455.
Article24. Wang X, LeMaire SA, Chen L, Shen YH, Gan Y, Bartsch H, Carter SA, Utama B, Ou H, Coselli JS, Wang XL. Increased collagen deposition and elevated expression of connective tissue growth factor in human thoracic aortic dissection. Circulation. 2006; 114(1 Suppl):I200–I205. PMID: 16820572.
Article25. Fan WH, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. Eur J Cell Biol. 2000; 79:915–923. PMID: 11152282.26. Yasuda Y, Nakamura J, Hamada Y, Nakayama M, Chaya S, Naruse K, Nakashima E, Kato K, Kamiya H, Hotta N. Role of PKC and TGF-beta receptor in glucose-induced proliferation of smooth muscle cells. Biochem Biophys Res Commun. 2001; 281:71–77. PMID: 11178962.27. Yasunari K, Kohno M, Kano H, Yokokawa K, Minami M, Yoshikawa J. Antioxidants improve impaired insulin-mediated glucose uptake and prevent migration and proliferation of cultured rabbit coronary smooth muscle cells induced by high glucose. Circulation. 1999; 99:1370–1378. PMID: 10077523.
Article28. Sakuma H, Yamamoto M, Okumura M, Kojima T, Maruyama T, Yasuda K. High glucose inhibits apoptosis in human coronary artery smooth muscle cells by increasing bcl-xL and bfl-1/A1. Am J Physiol Cell Physiol. 2002; 283:C422–C428. PMID: 12107051.29. Crean JK, Finlay D, Murphy M, Moss C, Godson C, Martin F, Brady HR. The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J Biol Chem. 2002; 277:44187–44194. PMID: 12218048.
Article30. Chung AC, Zhang H, Kong YZ, Tan JJ, Huang XR, Kopp JB, Lan HY. Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc Nephrol. 2010; 21:249–260. PMID: 19959709.31. Liu Y, Meyer C, Müller A, Herweck F, Li Q, Müllenbach R, Mertens PR, Dooley S, Weng HL. IL-13 induces connective tissue growth factor in rat hepatic stellate cells via TGF-β-independent Smad signaling. J Immunol. 2011; 187:2814–2823. PMID: 21804025.
Article32. Lee DH, Kim JE, Kang YJ. Insulin Like Growth Factor Binding Protein-5 Regulates Excessive Vascular Smooth Muscle Cell Proliferation in Spontaneously Hypertensive Rats via ERK 1/2 Phosphorylation. Korean J Physiol Pharmacol. 2013; 17:157–162. PMID: 23626478.
Article33. Osawa M, Itoh S, Ohta S, Huang Q, Berk BC, Marmarosh NL, Che W, Ding B, Yan C, Abe J. ERK1/2 associates with the c-Met-binding domain of growth factor receptor-bound protein 2 (Grb2)-associated binder-1 (Gab1): role in ERK1/2 and early growth response factor-1 (Egr-1) nuclear accumulation. J Biol Chem. 2004; 279:29691–29699. PMID: 15078886.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fluvastatin inhibits advanced glycation end products-induced proliferation, migration, and extracellular matrix accumulation in vascular smooth muscle cells by targeting connective tissue growth factor
- Morphological Analysis of Intimal Hyperplasia in Allografted Aorta of Rat
- Expression of growth factor, extracellular matrix and antioxidant (N-acetylcysteine) effect in TGF beta1 treated rat lens system
- Effect of high glucose on synthesis and gene expression of collagen and fibronectin in cultured vascular smooth muscle cells
- Proliferative and Synthetic Responses of Airway Smooth Muscle in Asthma