Korean J Physiol Pharmacol.
2018 Mar;22(2):193-201. 10.4196/kjpp.2018.22.2.193.
Fluvastatin inhibits advanced glycation end products-induced proliferation, migration, and extracellular matrix accumulation in vascular smooth muscle cells by targeting connective tissue growth factor
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, Yeungnam University, Daegu 42415, Korea. yjkang@med.yu.ac.kr
- 2School of Food Science and Biotechnology, Kyungpook National University, Daegu 41566, Korea.
- KMID: 2410109
- DOI: http://doi.org/10.4196/kjpp.2018.22.2.193
Abstract
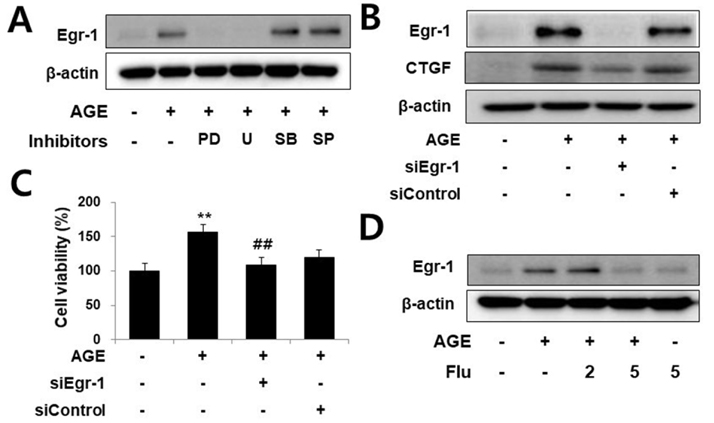
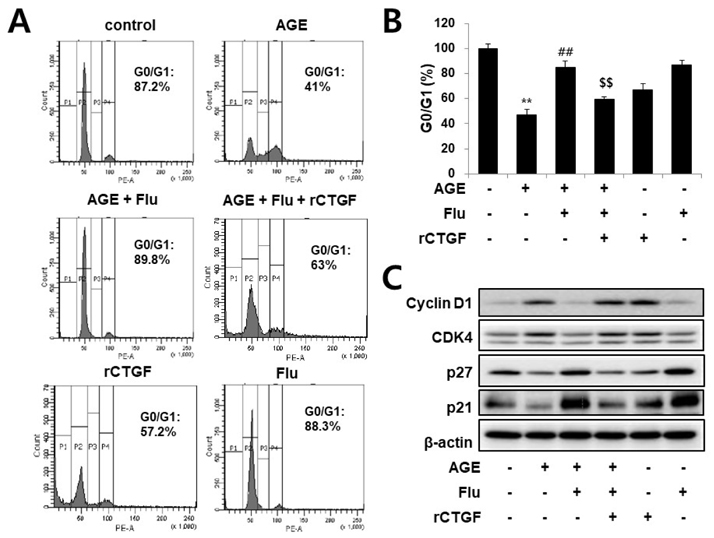
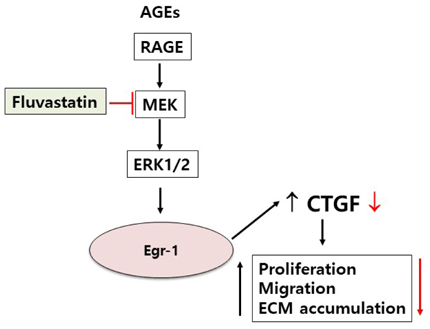
- Connective tissue growth factor (CTGF) is a novel fibrotic mediator, which is considered to mediate fibrosis through extracellular matrix (ECM) synthesis in diabetic cardiovascular complications. Statins have significant immunomodulatory effects and reduce vascular injury. We therefore examined whether fluvastatin has anti-fibrotic effects in vascular smooth muscle cells (VSMCs) and elucidated its putative transduction signals. We show that advanced glycation end products (AGEs) stimulated CTGF mRNA and protein expression in a time-dependent manner. AGE-induced CTGF expression was mediated via ERK1/2, JNK, and Egr-1 pathways, but not p38; consequently, cell proliferation and migration and ECM accumulation were regulated by CTGF signaling pathway. AGE-stimulated VSMC proliferation, migration, and ECM accumulation were blocked by fluvastatin. However, the inhibitory effect of fluvastatin was restored by administration of CTGF recombinant protein. AGE-induced VSMC proliferation was dependent on cell cycle arrest, thereby increasing G1/G0 phase. Fluvastatin repressed cell cycle regulatory genes cyclin D1 and Cdk4 and augmented cyclin-dependent kinase inhibitors p27 and p21 in AGE-induced VSMCs. Taken together, fluvastatin suppressed AGE-induced VSMC proliferation, migration, and ECM accumulation by targeting CTGF signaling mechanism. These findings might be evidence for CTGF as a potential therapeutic target in diabetic vasculature complication.
Keyword
MeSH Terms
-
Cell Cycle
Cell Cycle Checkpoints
Cell Proliferation
Connective Tissue Growth Factor*
Connective Tissue*
Cyclin D1
Extracellular Matrix*
Fibrosis
Genes, Regulator
Glycosylation End Products, Advanced
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Muscle, Smooth, Vascular*
Phosphotransferases
RNA, Messenger
Vascular System Injuries
Connective Tissue Growth Factor
Cyclin D1
Glycosylation End Products, Advanced
Phosphotransferases
RNA, Messenger
Figure
Reference
-
1. Andreea SI, Marieta C, Anca D. AGEs and glucose levels modulate type I and III procollagen mRNA synthesis in dermal fibroblasts cells culture. Exp Diabetes Res. 2008; 2008:473603.
Article2. Makita Z, Bucala R, Rayfield EJ, Friedman EA, Kaufman AM, Korbet SM, Barth RH, Winston JA, Fuh H, Manogue KR, et al. Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet. 1994; 343:1519–1522.
Article3. Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004; 17:31S–38S.
Article4. Reddy GK. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004; 68:132–142.5. Throckmorton DC, Brogden AP, Min B, Rasmussen H, Kashgarian M. PDGF and TGF-beta mediate collagen production by mesangial cells exposed to advanced glycosylation end products. Kidney Int. 1995; 48:111–117.6. Rupérez M, Lorenzo O, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003; 108:1499–1505.
Article7. Leask A, Holmes A, Abraham DJ. Connective tissue growth factor: a new and important player in the pathogenesis of fibrosis. Curr Rheumatol Rep. 2002; 4:136–142.
Article8. Crean JK, Finlay D, Murphy M, Moss C, Godson C, Martin F, Brady HR. The role of p42/44 MAPK and protein kinase B in connective tissue growth factor induced extracellular matrix protein production, cell migration, and actin cytoskeletal rearrangement in human mesangial cells. J Biol Chem. 2002; 277:44187–44194.
Article9. Ha YM, Lee DH, Kim M, Kang YJ. High glucose induces connective tissue growth factor expression and extracellular matrix accumulation in rat aorta vascular smooth muscle cells via extracellular signal-regulated kinase 1/2. Korean J Physiol Pharmacol. 2013; 17:307–314.
Article10. Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007; 370:1829–1839.11. Dechend R, Müller D, Park JK, Fiebeler A, Haller H, Luft FC. Statins and angiotensin II-induced vascular injury. Nephrol Dial Transplant. 2002; 17:349–353.
Article12. Ha YM, Nam JO, Kang YJ. Pitavastatin regulates ang II induced proliferation and migration via IGFBP-5 in VSMC. Korean J Physiol Pharmacol. 2015; 19:499–506.
Article13. Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J. 2007; 21:3355–3368.
Article14. Furfaro AL, Sanguineti R, Storace D, Monacelli F, Puzzo A, Pronzato MA, Odetti P, Traverso N. Metalloproteinases and advanced glycation end products: coupled navigation in atherosclerotic plaque pathophysiology? Exp Clin Endocrinol Diabetes. 2012; 120:586–590.
Article15. Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008; 4:575–596.16. Francis-Sedlak ME, Moya ML, Huang JJ, Lucas SA, Chandrasekharan N, Larson JC, Cheng MH, Brey EM. Collagen glycation alters neovascularization in vitro and in vivo. Microvasc Res. 2010; 80:3–9.
Article17. Yoon SJ, Yoon YW, Lee BK, Kwon HM, Hwang KC, Kim M, Chang W, Hong BK, Lee YH, Park SJ, Min PK, Rim SJ. Potential role of HMG CoA reductase inhibitor on oxidative stress induced by advanced glycation endproducts in vascular smooth muscle cells of diabetic vasculopathy. Exp Mol Med. 2009; 41:802–811.
Article18. Zhou Z, Wang K, Penn MS, Marso SP, Lauer MA, Forudi F, Zhou X, Qu W, Lu Y, Stern DM, Schmidt AM, Lincoff AM, Topol EJ. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003; 107:2238–2243.
Article19. Barreto SC, Ray A, Ag Edgar P. Biological characteristics of CCN proteins in tumor development. J BUON. 2016; 21:1359–1367.20. Mendes FA, Coelho Aguiar JM, Kahn SA, Reis AH, Dubois LG, Romão LF, Ferreira LS, Chneiweiss H, Moura Neto V, Abreu JG. Connective-tissue growth factor (CTGF/CCN2) induces astrogenesis and fibronectin expression of embryonic neural cells in vitro. PLoS One. 2015; 10:e0133689.
Article21. Ponticos M. Connective tissue growth factor (CCN2) in blood vessels. Vascul Pharmacol. 2013; 58:189–193.
Article22. Wu YL, Li HY, Zhao XP, Jiao JY, Tang DX, Yan LJ, Wan Q, Pan CB. Mesenchymal stem cell-derived CCN2 promotes the proliferation, migration and invasion of human tongue squamous cell carcinomacells. Cancer Sci. 2017; 108:897–909.23. Hu X, Wang H, Liu J, Fang X, Tao K, Wang Y, Li N, Shi J, Wang Y, Ji P, Cai W, Bai X, Zhu X, Han J, Hu D. The role of ERK and JNK signaling in connective tissue growth factor induced extracellular matrix protein production and scar formation. Arch Dermatol Res. 2013; 305:433–445.
Article24. Lin CH, Shih CH, Tseng CC, Yu CC, Tsai YJ, Bien MY, Chen BC. CXCL12 induces connective tissue growth factor expression in human lung fibroblasts through the Rac1/ERK, JNK, and AP-1 pathways. PLoS One. 2014; 9:e104746.
Article25. Liu B, Yu J, Taylor L, Zhou X, Polgar P. Microarray and phosphokinase screenings leading to studies on ERK and JNK regulation of connective tissue growth factor expression by angiotensin II 1a and bradykinin B2 receptors in Rat1 fibroblasts. J Cell Biochem. 2006; 97:1104–1120.
Article26. Huang J, Huang H, Wu M, Li J, Xie H, Zhou H, Liao E, Peng Y. Connective tissue growth factor induces osteogenic differentiation of vascular smooth muscle cells through ERK signaling. Int J Mol Med. 2013; 32:423–429.
Article27. Simó S, Pujadas L, Segura MF, La Torre A, Del Río JA, Ureña JM, Comella JX, Soriano E. Reelin induces the detachment of postnatal subventricular zone cells and the expression of the Egr-1 through Erk1/2 activation. Cereb Cortex. 2007; 17:294–303.
Article28. Zhang J, Song J, Xu J, Chen X, Yin P, Lv X, Wang X. ERK1/2-Egr-1 signaling pathway-mediated protective effects of electroacupuncture in a mouse model of myocardial ischemia-reperfusion. Evid Based Complement Alternat Med. 2014; 2014:253075.
Article29. Hwang AR, Han JH, Lim JH, Kang YJ, Woo CH. Fluvastatin inhibits AGE-induced cell proliferation and migration via an ERK5-dependent Nrf2 pathway in vascular smooth muscle cells. PLoS One. 2017; 12:e0178278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphological Analysis of Intimal Hyperplasia in Allografted Aorta of Rat
- Alagebrium Chloride, a Novel Advanced Glycation End-Product Cross Linkage Breaker, Inhibits Neointimal Proliferation in a Diabetic Rat Carotid Balloon Injury Model
- High Glucose Induces Connective Tissue Growth Factor Expression and Extracellular Matrix Accumulation in Rat Aorta Vascular Smooth Muscle Cells Via Extracellular Signal-Regulated Kinase 1/2
- The effect of advanced glycation and products on the proliferation of vascular smooth muscle cell
- Effect of Advanced Glycation End Products on Rat Aortic Vascular Smooth Muscle Cells