Korean J Physiol Pharmacol.
2012 Feb;16(1):49-57. 10.4196/kjpp.2012.16.1.49.
Acute Toxicity and General Pharmacological Action of QGC EXT
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- 2Department of Pharmacognosy, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 2285440
- DOI: http://doi.org/10.4196/kjpp.2012.16.1.49
Abstract
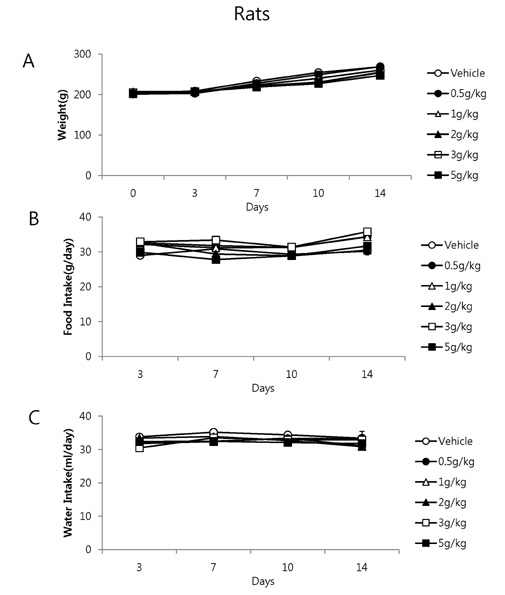
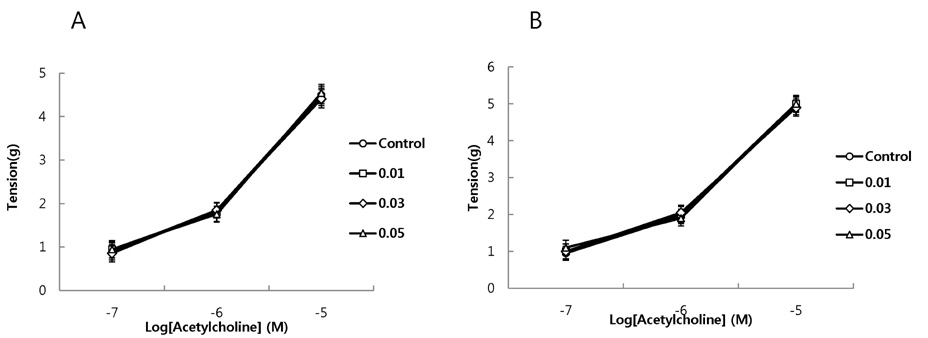
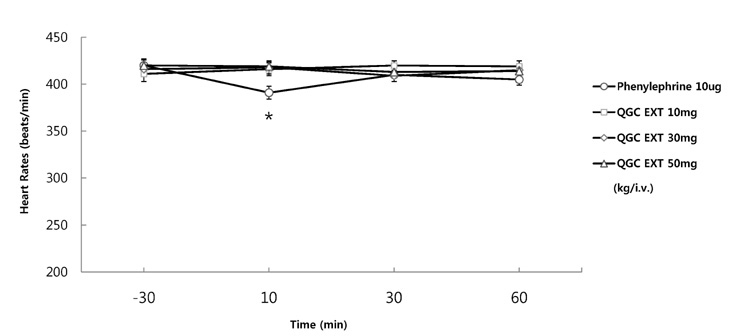
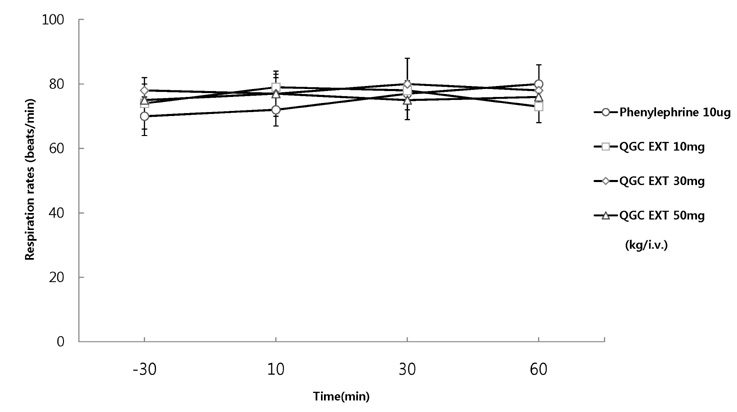
- It has been shown that QGC isolated and purified from Rumecis folium found protective effects of gastritis and esophagitis which EXT is an ethanol extract of it. We examined acute toxicity and the general pharmacological action of QGC EXT to search for any side effects of it in rats, mice, guinea pigs, and cats. In a single dose toxicity study, QGC EXT didn't show toxicological effects in rats and mice, and the LD50 was over 5 g/kg in both animals, and there were also no changes in weight, feed and water intake during these toxicological experimental periods. We examined the general pharmacological action on central controlled behavior responses, and peripheral organs including blood pressure, heart rate, respiration and gastrointestinal system, We found that there were no significant changes in body temperature, locomotors activity, stereotyped behaviors, sleeping time, and convulsion. In other studies, writhing reaction, normal body temperature, there did not appear to be any changes. The large intestine movement and electrical field stimulation-induced contraction was not changes by its EXT. In addition, the influences on blood pressure, heart rates, and respiration by QGC EXT were not found. These results indicate that QGC EXT may be very safe as a new drug, since its LD50 was very high over 5 g/kg and any side effects were not found.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Inhibitory Effects of ECQ on Indomethacin-Induced Gastric Damage in Rats
Juho Jung, Yoonjin Nam, Uy Dong Sohn
Korean J Physiol Pharmacol. 2012;16(6):399-404. doi: 10.4196/kjpp.2012.16.6.399.
Reference
-
1. Haenen GR, Paquay JB, Korthouwer RE, Bast A. Peroxynitrite scavenging by flavonoids. Biochem Biophys Res Commun. 1997. 236:591–593.2. Diplock AT. Defense against reactive oxygen species. Free Radic Res. 1998. 29:463–467.3. Heijnen CG, Haenen GR, van Acker FA, van der Vijgh WJ, Bast A. Flavonoids as peroxynitrite scavengers: the role of the hydroxyl groups. Toxicol In Vitro. 2001. 15:3–6.4. Min YS, Lee SE, Hong ST, Kim HS, Choi BC, Sim SS, Whang WK, Sohn UD. The Inhibitory Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Gastritis and Reflux Esophagitis in Rats. Korean J Physiol Pharmacol. 2009. 13:295–300.5. Yan XM, Joo MJ, Lim JC, Whang WK, Sim SS, Im C, Kim HR, Lee SY, Kim IK, Sohn UD. The effect of quercetin-3-O-β-D-glucuronopyranoside on indomethacin-induced gastric damage in rats via induction of mucus secretion and down-regulation of ICAM-1 expression. Arch Pharm Res. 2011. 34:1527–1534.6. Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968. 13:222–257.7. Lee EB. Pharmacological approach of crude drugs. Yakhak Hoeji. 1975. 19:53–59.8. Dunham NW, Miya TS, Eewards LD. The pharmacological activity of a series of basic esters of mono- and dialkylmalonic acids. J Am Pharm Assoc Am Pharm Assoc (Baltim). 1957. 46:64–66.9. Koster R, Anderson M, de Beer EJ. Acetic acid for analgesic. 210 screening. Fed Proc. 1959. 18:412–418.10. Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957. 111:409–419.11. Araki Y, Ueki S. Changes in sensitivity to convulsion in mice with olfactory bulb ablation. Jpn J Pharmacol. 1972. 22:447–456.12. Swinyard EA, Brown WC, Goodman LS. Comparative assays of antiepileptic drugs in mice and rats. J Pharmacol Exp Ther. 1952. 106:319–330.13. Sohn UD, Harnett KM, De Petris G, Behar J, Biancani P. Distinct muscarinic receptors, G proteins and phospholipases in esophageal and lower esophageal sphincter circular muscle. J Pharmacol Exp Ther. 1993. 267:1205–1214.14. Biancani P, Hillemeier C, Bitar KN, Makhlouf GM. Contraction mediated by Ca2+ influx in esophageal muscle and by Ca2+ release in the LES. Am J Physiol. 1987. 253:G760–G766.15. Tomlinson TM, Akerele O. Medicinal plants their role in health and biodiversity. 1998. Philadelphia: University of Pennsylvania Press.16. Sohn UD, Cho JH, Song HJ, Sun YH, Hwang WK. Protective effects of Quercetin-3-O-β-D-glucuronopyranoside (QGC) on ethanol-induced cell damage involve inhibitions of ROS generation and downstream activation of the ERK in feline esophageal epithelial cells. M1903. 2009. In : 2009 Digestive Disease Week; May 30th-June 4th; McCormick Place, Chicago, IL, USA.17. Alvarez A, Pomar F, Sevilla , Montero MJ. Gastric antisecretory and antiulcer activities of an ethanolic extract of Bidens pilosa L. var. radiata Schult. Bip. J Ethnopharmacol. 1999. 67:333–340.18. Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992. 51:Suppl 1. 59–67.19. Murakami S, Muramatsu M, Otomo S. Inhibition of gastric H+, K(+)-ATPase by quercetin. J Enzyme Inhib. 1992. 5:293–298.20. Parmar NS, Hennings G. The gastric antisecretory activity of 3-methoxy-5,7,3'4'-tetrahydroxyflavan (ME)--a specific histidine decarboxylase inhibitor in rats. Agents Actions. 1984. 15:143–145.21. Gambhir SS, Goel RK, Das Gupta G. Anti-inflammatory & anti-ulcerogenic activity of amentoflavone. Indian J Med Res. 1987. 85:689–693.22. Gerritsen ME, Carley WW, Ranges GE, Shen CP, Phan SA, Ligon GF, Perry CA. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995. 147:278–292.23. Panés J, Gerritsen ME, Anderson DC, Miyasaka M, Granger DN. Apigenin inhibits tumor necrosis factor-induced intercellular adhesion molecule-1 upregulation in vivo. Microcirculation. 1996. 3:279–286.24. Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996. 20:933–956.25. Gordon MH, Roedig-Penman A. Antioxidant activity of quercetin and myricetin in liposomes. Chem Phys Lipids. 1998. 97:79–85.26. Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001. 30:433–446.27. Gerritsen ME, Carley WW, Ranges GE, Shen CP, Phan SA, Ligon GF, Perry CA. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995. 147:278–292.28. Lee SE, Jang HS, Song HJ, Hwang WK, Sohn UD. M1904 Downstream Signal Transduction Induced By Interleukin-1 Beta-Stimulated ROS Generation and Anti-Oxidative Effects of Quercetin-3-O-[beta]-D-Glucuronopyranoside (QGC) in Feline Esophageal Epithelial Cell. Gastroenterology. 2009. 136:A442–A443.29. Loomis TA, Hayes AW. Loomis's essentials of toxicology. 1996. 4th ed. California: Academic Press.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastroprotective Effect of the Three Glucuronopyranoside Flavonoids in Rats
- The Inhibitory Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Gastritis and Reflux Esophagitis in Rats
- Anti-Oxidative and Anti-Inflammatory Effects of QGC in Cultured Feline Esophageal Epithelial Cells
- Sodium-Glucose Cotransporter 2 Inhibitors: Mechanisms of Action and Various Effects
- Receptor-Mediated Muscle Homeostasis as a Target for Sarcopenia Therapeutics