Korean J Physiol Pharmacol.
2013 Feb;17(1):81-87. 10.4196/kjpp.2013.17.1.81.
Anti-Oxidative and Anti-Inflammatory Effects of QGC in Cultured Feline Esophageal Epithelial Cells
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- KMID: 1432767
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.81
Abstract
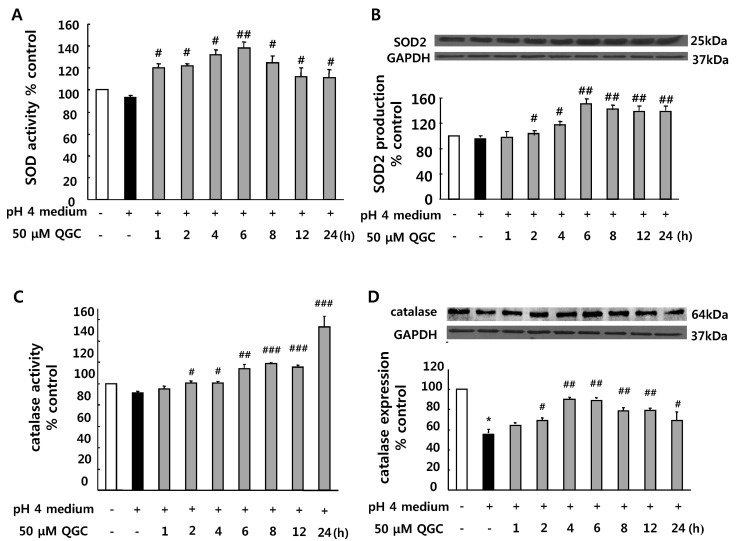
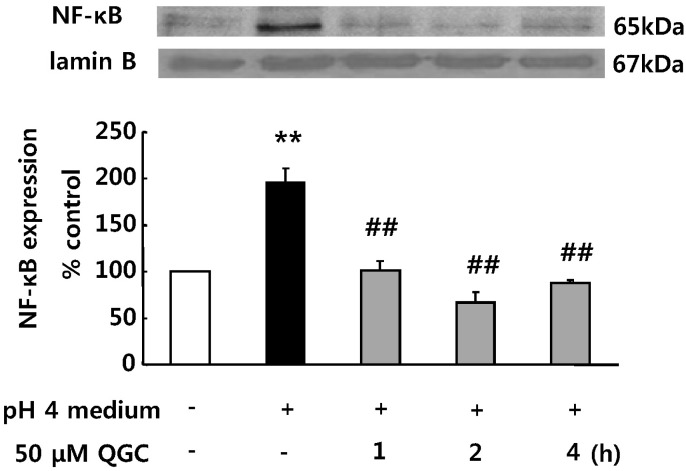
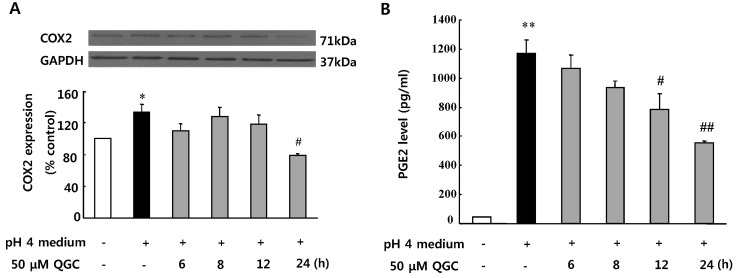
- Quercetin-3-O-beta-D-glucuronopyranoside (QGC) is a flavonoid glucoside extracted from Rumex Aquaticus Herba. In the present study, anti-oxidative and anti-inflammatory effects of QGC were tested in vitro. Epithelial cells obtained from cat esophagus were cultured. When the cells were exposed to acid for 2 h, cell viability was decreased to 36%. Pretreatment with 50 microM QGC for 2 h prevented the reduction in cell viability. QGC also inhibited the productions of intracellular ROS by inflammatory inducers such as acid, lipopolysaccharide, indomethacin and ethanol. QGC significantly increased the activities of superoxide dismutase (SOD) and catalase, and also induced the expression of SOD2, while it restored the decrease of catalase expression in cells exposed to acid. QGC inhibited NF-kappaB translocation, cyclooxygenase-2 expression and PGE2 secretion in cells exposed to acid, which plays an important role in the pathogenesis of esophagitis. The data suggest that QGC may well be one of the promising substances to attenuate oxidative epithelial cell injury and inflammatory signaling in esophagus inflammation.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of C18 Fatty Acids on Intracellular Ca2+ Mobilization and Histamine Release in RBL-2H3 Cells
Myung Chul Kim, Min Gyu Kim, Young Soo Jo, Ho Sun Song, Tae In Eom, Sang Soo Sim
Korean J Physiol Pharmacol. 2014;18(3):241-247. doi: 10.4196/kjpp.2014.18.3.241.
Reference
-
1. Aviram M. Review of human studies on oxidative damage and antioxidant protection related to cardiovascular diseases. Free Radic Res. 2000; 33(Suppl):S85–S97. PMID: 11191279.2. Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994; 344:721–724. PMID: 7915779.
Article3. De Magalhaes JP, Church GM. Cells discover fire: employing reactive oxygen species in development and consequences for aging. Exp Gerontol. 2006; 41:1–10. PMID: 16226003.4. Kim KC, Lee C. Curcumin induces downregulation of E2f4 expression and apoptotic cell death in HCT116 human colon cancer cells; Involvement of reactive oxygen species. Korean J Physiol Pharmacol. 2010; 14:391–397. PMID: 21311680.
Article5. Menon SG, Goswami PC. A redox cycle within the cell cycle: ring in the old with the new. Oncogene. 2007; 26:1101–1109. PMID: 16924237.
Article6. Chen KC, Zhou Y, Zhang W, Lou MF. Control of PDGF-induced reactive oxygen species (ROS) generation and signal transduction in human lens epithelial cells. Mol Vis. 2007; 13:374–387. PMID: 17392688.7. Wang L, Tu YC, Lian TW, Hung JT, Yen JH, Wu MJ. Distinctive antioxidant and antiinflammatory effects of flavonols. J Agric Food Chem. 2006; 54:9798–9804. PMID: 17177504.
Article8. Hernández-Ledesma B, Hsieh CC, de Lumen BO. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009; 390:803–808. PMID: 19836349.
Article9. Yamaguchi T, Yoshida N, Tomatsuri N, Takayama R, Katada K, Takagi T, Ichikawa H, Naito Y, Okanoue T, Yoshikawa T. Cytokine-induced neutrophil accumulation in the pathogenesis of acute reflux esophagitis in rats. Int J Mol Med. 2005; 16:71–77. PMID: 15942680.
Article10. Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010; 58:85–90. PMID: 20399614.
Article11. Itoh T, Hamada N, Terazawa R, Ito M, Ohno K, Ichihara M, Nozawa Y, Ito M. Molecular hydrogen inhibits lipopolysaccharide/interferon γ-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem Biophys Res Commun. 2011; 411:143–149. PMID: 21723254.
Article12. Hang CH, Shi JX, Li JS, Li WQ, Yin HX. Up-regulation of intestinal nuclear factor kappa B and intercellular adhesion molecule-1 following traumatic brain injury in rats. World J Gastroenterol. 2005; 11:1149–1154. PMID: 15754395.
Article13. Al-Ashy R, Chakroun I, El-Sabban ME, Homaidan FR. The role of NF-kappaB in mediating the anti-inflammatory effects of IL-10 in intestinal epithelial cells. Cytokine. 2006; 36:1–8. PMID: 17161612.14. Odeleye OE, Eskelson CD, Mufti SI, Watson RR. Vitamin E inhibition of lipid peroxidation and ethanol-mediated promotion of esophageal tumorigenesis. Nutr Cancer. 1992; 17:223–234. PMID: 1437642.
Article15. Dimayuga FO, Wang C, Clark JM, Dimayuga ER, Dimayuga VM, Bruce-Keller AJ. SOD1 overexpression alters ROS production and reduces neurotoxic inflammatory signaling in microglial cells. J Neuroimmunol. 2007; 182:89–99. PMID: 17097745.
Article16. Sugino N. The role of oxygen radical-mediated signaling pathways in endometrial function. Placenta. 2007; 28(Suppl A):S133–S136. PMID: 17291583.
Article17. Yamamoto T, Lewis J, Wataha J, Dickinson D, Singh B, Bollag WB, Ueta E, Osaki T, Athar M, Schuster G, Hsu S. Roles of catalase and hydrogen peroxide in green tea polyphenol-induced chemopreventive effects. J Pharmacol Exp Ther. 2004; 308:317–323. PMID: 14569057.
Article18. Wetscher GJ, Hinder RA, Klingler P, Gadenstätter M, Perdikis G, Hinder PR. Reflux esophagitis in humans is a free radical event. Dis Esophagus. 1997; 10:29–32. PMID: 9079270.
Article19. Li Y, Wo JM, Su RR, Ray MB, Martin RC. Loss of manganese superoxide dismutase expression and activity in rat esophagus with external esophageal perfusion. Surgery. 2007; 141:359–367. PMID: 17349848.
Article20. Moreira AJ, Fraga C, Alonso M, Collado PS, Zetller C, Marroni C, Marroni N, González-Gallego J. Quercetin prevents oxidative stress and NF-kappaB activation in gastric mucosa of portal hypertensive rats. Biochem Pharmacol. 2004; 68:1939–1946. PMID: 15476665.21. Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001; 74:418–425. PMID: 11566638.
Article22. Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000; 28:463–499. PMID: 10699758.
Article23. Chow JM, Shen SC, Huan SK, Lin HY, Chen YC. Quercetin, but not rutin and quercitrin, prevention of H2O2-induced apoptosis via anti-oxidant activity and heme oxygenase 1 gene expression in macrophages. Biochem Pharmacol. 2005; 69:1839–1851. PMID: 15876423.24. Kahraman A, Erkasap N, Käken T, Serteser M, Aktepe F, Erkasap S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology. 2003; 183:133–142. PMID: 12504347.
Article25. Milkes D, Gerson LB, Triadafilopoulos G. Complete elimination of reflux symptoms does not guarantee normalization of intraesophageal and intragastric pH in patients with gastroesophageal reflux disease (GERD). Am J Gastroenterol. 2004; 99:991–996. PMID: 15180715.
Article26. Song HJ, Shin CY, Oh TY, Min YS, Park ES, Sohn UD. Eupatilin with heme oxygenase-1-inducing ability protects cultured feline esophageal epithelial cells from cell damage caused by indomethacin. Biol Pharm Bull. 2009; 32:589–596. PMID: 19336889.
Article27. Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2',7'-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003; 65:1575–1582. PMID: 12754093.
Article28. Zarling EJ. A review of reflux esophagitis around the world. World J Gastroenterol. 1998; 4:280–284. PMID: 11819299.
Article29. Cappell MS. Clinical presentation, diagnosis, and management of gastroesophageal reflux disease. Med Clin North Am. 2005; 89:243–291. PMID: 15656927.
Article30. Farhadi A, Fields J, Banan A, Keshavarzian A. Reactive oxygen species: are they involved in the pathogenesis of GERD, Barrett's esophagus, and the latter's progression toward esophageal cancer? Am J Gastroenterol. 2002; 97:22–26. PMID: 11808965.
Article31. Bell RC, Freeman KD. Clinical and pH-metric outcomes of transoral esophagogastric fundoplication for the treatment of gastroesophageal reflux disease. Surg Endosc. 2011; 25:1975–1984. PMID: 21140170.
Article32. Jiménez P, Piazuelo E, Sánchez MT, Ortego J, Soteras F, Lanas A. Free radicals and antioxidant systems in reflux esophagitis and Barrett's esophagus. World J Gastroenterol. 2005; 11:2697–2703. PMID: 15884106.
Article33. De Souza LF, Barreto F, da Silva EG, Andrades ME, Guimarães EL, Behr GA, Moreira JC, Bernard EA. Regulation of LPS stimulated ROS production in peritoneal macrophages from alloxan-induced diabetic rats: involvement of high glucose and PPARgamma. Life Sci. 2007; 81:153–159. PMID: 17532345.34. Graziani G, D'Argenio G, Tuccillo C, Loguercio C, Ritieni A, Morisco F, Del Vecchio Blanco C, Fogliano V, Romano M. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut. 2005; 54:193–200. PMID: 15647180.35. McCarroll JA, Phillips PA, Park S, Doherty E, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cell activation by ethanol and acetaldehyde: is it mediated by the mitogenctivated protein kinase signaling pathway? Pancreas. 2003; 27:150–160. PMID: 12883264.36. Min YS, Bai KL, Yim SH, Lee YJ, Song HJ, Kim JH, Ham I, Whang WK, Sohn UD. The effect of luteolin-7-O-beta-D-lucuronopyranoside on gastritis and esophagitis in rats. Arch Pharm Res. 2006; 29:484–489. PMID: 16833016.37. Lee JS, Oh TY, Ahn BO, Cho H, Kim WB, Kim YB, Surh YJ, Kim HJ, Hahm KB. Involvement of oxidative stress in experimentally induced reflux esophagitis and Barrett's esophagus: clue for the chemoprevention of esophageal carcinoma by antioxidants. Mutat Res. 2001; 480-481:189–200. PMID: 11506813.
Article38. Stickle RL, Epperly MW, Klein E, Bray JA, Greenberger JS. Prevention of irradiation-induced esophagitis by plasmid/liposome delivery of the human manganese superoxide dismutase transgene. Radiat Oncol Investig. 1999; 7:204–217.
Article39. Li YM, Chan HY, Huang Y, Chen ZY. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol Nutr Food Res. 2007; 51:546–554. PMID: 17440995.
Article40. Doronicheva N, Yasui H, Sakurai H. Chemical structure-dependent differential effects of flavonoids on the catalase activity as evaluated by a chemiluminescent method. Biol Pharm Bull. 2007; 30:213–217. PMID: 17268053.
Article41. Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004; 96:229–245. PMID: 15539763.
Article42. Reddy DB, Reddanna P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009; 381:112–117. PMID: 19351605.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Effect of Quercetin-3-O-beta-D-Glucuronopyranoside on Ethanol-induced Damage in Cultured Feline Esophageal Epithelial Cells
- Anti-Inflammatory Effects of Fermented Products with Avena sativa on RAW264.7 and HT-29 Cells via Inhibition of Inflammatory Mediators
- Human Amniotic Epithelial Cells Affect the Functions of Neutrophils
- Effects of Anti-Inflammatory Drugs on the Interleukin-1 beta-Induced Cyclooxygenase-2 Expression in Human Airway Epithelial Cells
- Gastroprotective Effect of the Three Glucuronopyranoside Flavonoids in Rats