Diabetes Metab J.
2013 Apr;37(2):106-112. 10.4093/dmj.2013.37.2.106.
A Systematic Review of Oxidative Stress and Safety of Antioxidants in Diabetes: Focus on Islets and Their Defense
- Affiliations
-
- 1Departments of Internal Medicine, Biochemistry and Cell Biology, Research Institute of Aging and Metabolism and World Class University Program, Kyungpook National University School of Medicine, Daegu, Korea. kpark@knu.ac.kr
- KMID: 2280711
- DOI: http://doi.org/10.4093/dmj.2013.37.2.106
Abstract
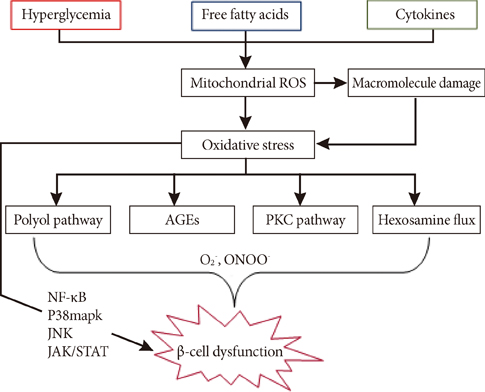
- A growing body of evidence suggests that hyperglycemia-induced oxidative stress plays an important role in diabetic complications, especially beta-cell dysfunction and failure. Under physiological conditions, reactive oxygen species serve as second messengers that facilitate signal transduction and gene expression in pancreatic beta-cells. However, under pathological conditions, an imbalance in redox homeostasis leads to aberrant tissue damage and beta-cell death due to a lack of antioxidant defense systems. Taking into account the vulnerability of islets to oxidative damage, induction of endogenous antioxidant enzymes or exogenous antioxidant administration has been proposed as a way to protect beta-cells against diabetic insults. Here, we consider recent insights into how the redox response becomes deregulated under diabetic conditions, as well as the therapeutic benefits of antioxidants, which may provide clues for developing strategies aimed at the treatment or prevention of diabetes associated with beta-cell failure.
Keyword
MeSH Terms
-
Antioxidants
Choristoma
Diabetes Complications
Gene Expression
Homeostasis
Nitric Oxide
Oxidation-Reduction
Oxidative Stress
Reactive Nitrogen Species
Reactive Oxygen Species
Second Messenger Systems
Signal Transduction
Superoxide Dismutase
Antioxidants
Nitric Oxide
Reactive Nitrogen Species
Reactive Oxygen Species
Superoxide Dismutase
Figure
Cited by 1 articles
-
The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
Diabetes Metab J. 2020;44(2):222-233. doi: 10.4093/dmj.2020.0053.
Reference
-
1. Porte D Jr. Clinical importance of insulin secretion and its interaction with insulin resistance in the treatment of type 2 diabetes mellitus and its complications. Diabetes Metab Res Rev. 2001. 17:181–188.2. Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003. 46:3–19.3. Rehman A, Nourooz-Zadeh J, Moller W, Tritschler H, Pereira P, Halliwell B. Increased oxidative damage to all DNA bases in patients with type II diabetes mellitus. FEBS Lett. 1999. 448:120–122.4. Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese type II diabetic patients. Diabetologia. 2002. 45:85–96.5. Mohamed AK, Bierhaus A, Schiekofer S, Tritschler H, Ziegler R, Nawroth PP. The role of oxidative stress and NF-kappaB activation in late diabetic complications. Biofactors. 1999. 10:157–167.6. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002. 23:599–622.7. Grankvist K, Marklund SL, Taljedal IB. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981. 199:393–398.8. Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996. 20:463–466.9. Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999. 48:2398–2406.10. Kubisch HM, Wang J, Bray TM, Phillips JP. Targeted overexpression of Cu/Zn superoxide dismutase protects pancreatic beta-cells against oxidative stress. Diabetes. 1997. 46:1563–1566.11. Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007. 56:1783–1791.12. Affourtit C, Jastroch M, Brand MD. Uncoupling protein-2 attenuates glucose-stimulated insulin secretion in INS-1E insulinoma cells by lowering mitochondrial reactive oxygen species. Free Radic Biol Med. 2011. 50:609–616.13. Saadeh M, Ferrante TC, Kane A, Shirihai O, Corkey BE, Deeney JT. Reactive oxygen species stimulate insulin secretion in rat pancreatic islets: studies using mono-oleoyl-glycerol. PLoS One. 2012. 7:e30200.14. LeRoith D, Taylor SI, Olefsky JM. Chapter 11. Glucose toxicity of the β-cell: cellular and molecular mechanisms. Diabetes mellitus: a fundamental and clinical text. 2000. 2nd ed. Philadelphia: Lippincott Williams & Wilkins;125–132.15. Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999. 48:927–932.16. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003. 17:24–38.17. Tang C, Han P, Oprescu AI, Lee SC, Gyulkhandanyan AV, Chan GN, Wheeler MB, Giacca A. Evidence for a role of superoxide generation in glucose-induced beta-cell dysfunction in vivo. Diabetes. 2007. 56:2722–2731.18. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001. 414:813–820.19. D'Alessandris C, Andreozzi F, Federici M, Cardellini M, Brunetti A, Ranalli M, Del Guerra S, Lauro D, Del Prato S, Marchetti P, Lauro R, Sesti G. Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. FASEB J. 2004. 18:959–961.20. Werstuck GH, Khan MI, Femia G, Kim AJ, Tedesco V, Trigatti B, Shi Y. Glucosamine-induced endoplasmic reticulum dysfunction is associated with accelerated atherosclerosis in a hyperglycemic mouse model. Diabetes. 2006. 55:93–101.21. Kaneto H, Xu G, Song KH, Suzuma K, Bonner-Weir S, Sharma A, Weir GC. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem. 2001. 276:31099–31104.22. Yan SD, Schmidt AM, Anderson GM, Zhang J, Brett J, Zou YS, Pinsky D, Stern D. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. J Biol Chem. 1994. 269:9889–9897.23. Jiang ZY, Woollard AC, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990. 268:69–71.24. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C: dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000. 49:1939–1945.25. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000. 404:787–790.26. Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004. 53:Suppl 1. S119–S124.27. Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005. 54:Suppl 2. S97–S107.28. Bakker SJ, RG IJ, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure. Atherosclerosis. 2000. 148:17–21.29. El-Assaad W, Joly E, Barbeau A, Sladek R, Buteau J, Maestre I, Pepin E, Zhao S, Iglesias J, Roche E, Prentki M. Glucolipotoxicity alters lipid partitioning and causes mitochondrial dysfunction, cholesterol, and ceramide deposition and reactive oxygen species production in INS832/13 ss-cells. Endocrinology. 2010. 151:3061–3073.30. Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, Bergamini E, Mosca F, Di Mario U, Del Prato S, Marchetti P. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes. 2002. 51:1437–1442.31. Elsner M, Gehrmann W, Lenzen S. Peroxisome-generated hydrogen peroxide as important mediator of lipotoxicity in insulin-producing cells. Diabetes. 2011. 60:200–208.32. Bindokas VP, Kuznetsov A, Sreenan S, Polonsky KS, Roe MW, Philipson LH. Visualizing superoxide production in normal and diabetic rat islets of Langerhans. J Biol Chem. 2003. 278:9796–9801.33. Igoillo-Esteve M, Marselli L, Cunha DA, Ladriere L, Ortis F, Grieco FA, Dotta F, Weir GC, Marchetti P, Eizirik DL, Cnop M. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia. 2010. 53:1395–1405.34. Shimabukuro M, Ohneda M, Lee Y, Unger RH. Role of nitric oxide in obesity-induced beta cell disease. J Clin Invest. 1997. 100:290–295.35. Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997. 46:1733–1742.36. Lortz S, Tiedge M. Sequential inactivation of reactive oxygen species by combined overexpression of SOD isoforms and catalase in insulin-producing cells. Free Radic Biol Med. 2003. 34:683–688.37. Moriscot C, Richard MJ, Favrot MC, Benhamou PY. Protection of insulin-secreting INS-1 cells against oxidative stress through adenoviral-mediated glutathione peroxidase overexpression. Diabetes Metab. 2003. 29(2 Pt 1):145–151.38. Benhamou PY, Moriscot C, Richard MJ, Beatrix O, Badet L, Pattou F, Kerr-Conte J, Chroboczek J, Lemarchand P, Halimi S. Adenovirus-mediated catalase gene transfer reduces oxidant stress in human, porcine and rat pancreatic islets. Diabetologia. 1998. 41:1093–1100.39. Gurgul E, Lortz S, Tiedge M, Jorns A, Lenzen S. Mitochondrial catalase overexpression protects insulin-producing cells against toxicity of reactive oxygen species and proinflammatory cytokines. Diabetes. 2004. 53:2271–2280.40. Xu B, Moritz JT, Epstein PN. Overexpression of catalase provides partial protection to transgenic mouse beta cells. Free Radic Biol Med. 1999. 27:830–837.41. Li X, Chen H, Epstein PN. Metallothionein and catalase sensitize to diabetes in nonobese diabetic mice: reactive oxygen species may have a protective role in pancreatic beta-cells. Diabetes. 2006. 55:1592–1604.42. Lee BW, Kwon SJ, Chae HY, Kang JG, Kim CS, Lee SJ, Yoo HJ, Kim JH, Park KS, Ihm SH. Dose-related cytoprotective effect of alpha-lipoic acid on hydrogen peroxide-induced oxidative stress to pancreatic beta cells. Free Radic Res. 2009. 43:68–77.43. Faust A, Burkart V, Ulrich H, Weischer CH, Kolb H. Effect of lipoic acid on cyclophosphamide-induced diabetes and insulitis in non-obese diabetic mice. Int J Immunopharmacol. 1994. 16:61–66.44. Busse E, Zimmer G, Schopohl B, Kornhuber B. Influence of alpha-lipoic acid on intracellular glutathione in vitro and in vivo. Arzneimittelforschung. 1992. 42:829–831.45. Koh EH, Lee WJ, Lee SA, Kim EH, Cho EH, Jeong E, Kim DW, Kim MS, Park JY, Park KG, Lee HJ, Lee IK, Lim S, Jang HC, Lee KH, Lee KU. Effects of alpha-lipoic acid on body weight in obese subjects. Am J Med. 2011. 124:85.46. Jacob S, Henriksen EJ, Tritschler HJ, Augustin HJ, Dietze GJ. Improvement of insulin-stimulated glucose-disposal in type 2 diabetes after repeated parenteral administration of thioctic acid. Exp Clin Endocrinol Diabetes. 1996. 104:284–288.47. Estrada DE, Ewart HS, Tsakiridis T, Volchuk A, Ramlal T, Tritschler H, Klip A. Stimulation of glucose uptake by the natural coenzyme alpha-lipoic acid/thioctic acid: participation of elements of the insulin signaling pathway. Diabetes. 1996. 45:1798–1804.48. Bast A, Haenen GR. Lipoic acid: a multifunctional antioxidant. Biofactors. 2003. 17:207–213.49. Schroeder MM, Belloto RJ Jr, Hudson RA, McInerney MF. Effects of antioxidants coenzyme Q10 and lipoic acid on interleukin-1 beta-mediated inhibition of glucose-stimulated insulin release from cultured mouse pancreatic islets. Immunopharmacol Immunotoxicol. 2005. 27:109–122.50. Coskun O, Kanter M, Korkmaz A, Oter S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res. 2005. 51:117–123.51. Meghana K, Sanjeev G, Ramesh B. Curcumin prevents streptozotocin-induced islet damage by scavenging free radicals: a prophylactic and protective role. Eur J Pharmacol. 2007. 577:183–191.52. Kim HY, Kim K. Protective effect of ginseng on cytokine-induced apoptosis in pancreatic beta-cells. J Agric Food Chem. 2007. 55:2816–2823.53. Kim EK, Kwon KB, Song MY, Seo SW, Park SJ, Ka SO, Na L, Kim KA, Ryu DG, So HS, Park R, Park JW, Park BH. Genistein protects pancreatic beta cells against cytokine-mediated toxicity. Mol Cell Endocrinol. 2007. 278:18–28.54. Cai EP, Lin JK. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J Agric Food Chem. 2009. 57:9817–9827.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Metabolic Syndrome and Oxidative Stress, Antioxidants
- Colored Foods and Diabetes
- Can antioxidants be effective therapeutics for type 2 diabetes?
- Oxidative Stress in Pancreatic Islet beta-cells Exposed to High Glucose Concentration
- Effects of Antioxidants on Ethidium Bromide-induced Inhibition of Insulin Secretion in Rat Pancreatic Islets