Yeungnam Univ J Med.
2021 Apr;38(2):83-94. 10.12701/yujm.2020.00563.
Can antioxidants be effective therapeutics for type 2 diabetes?
- Affiliations
-
- 1Department of Physiology and Smart-aging Convergence Research Center, Yeungnam University College of Medicine, Daegu, Korea
- KMID: 2515182
- DOI: http://doi.org/10.12701/yujm.2020.00563
Abstract
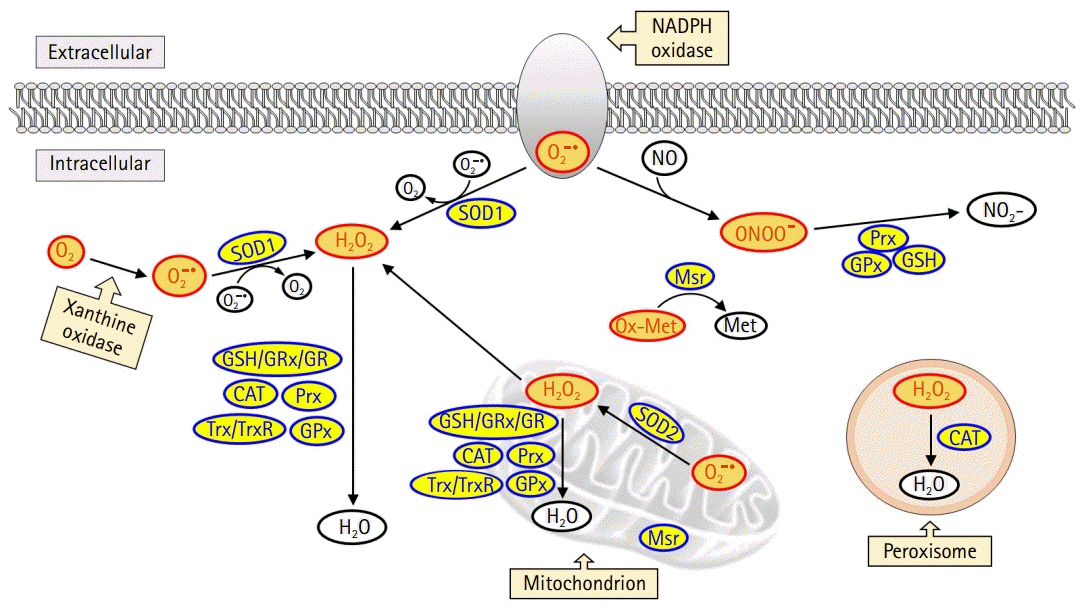
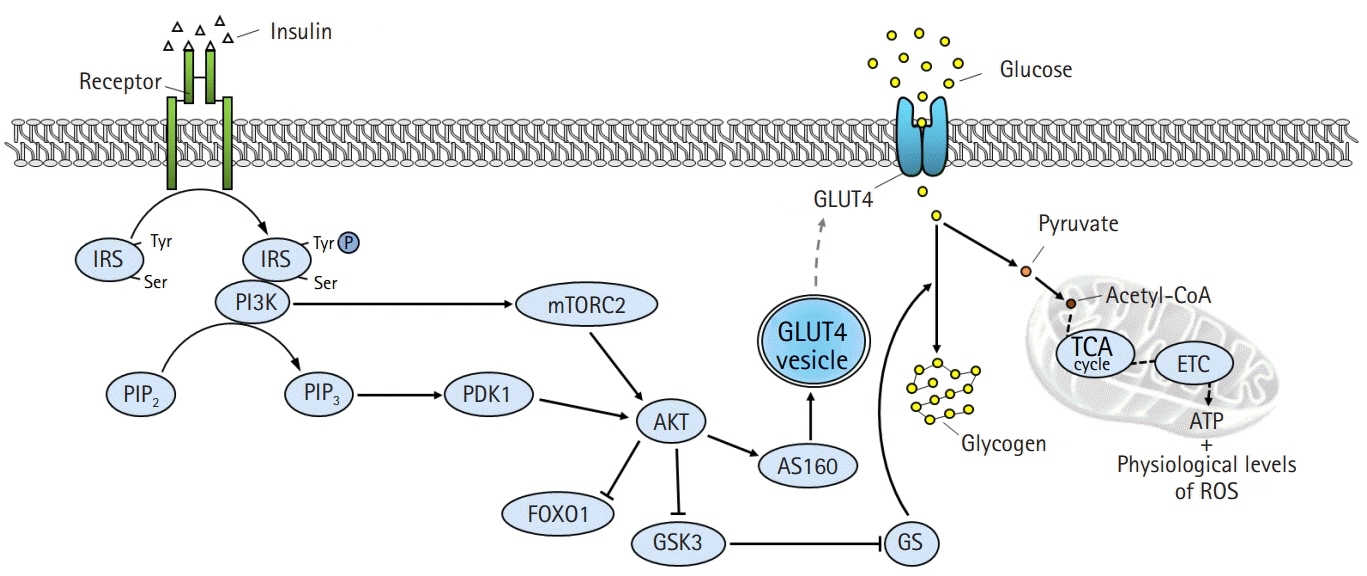
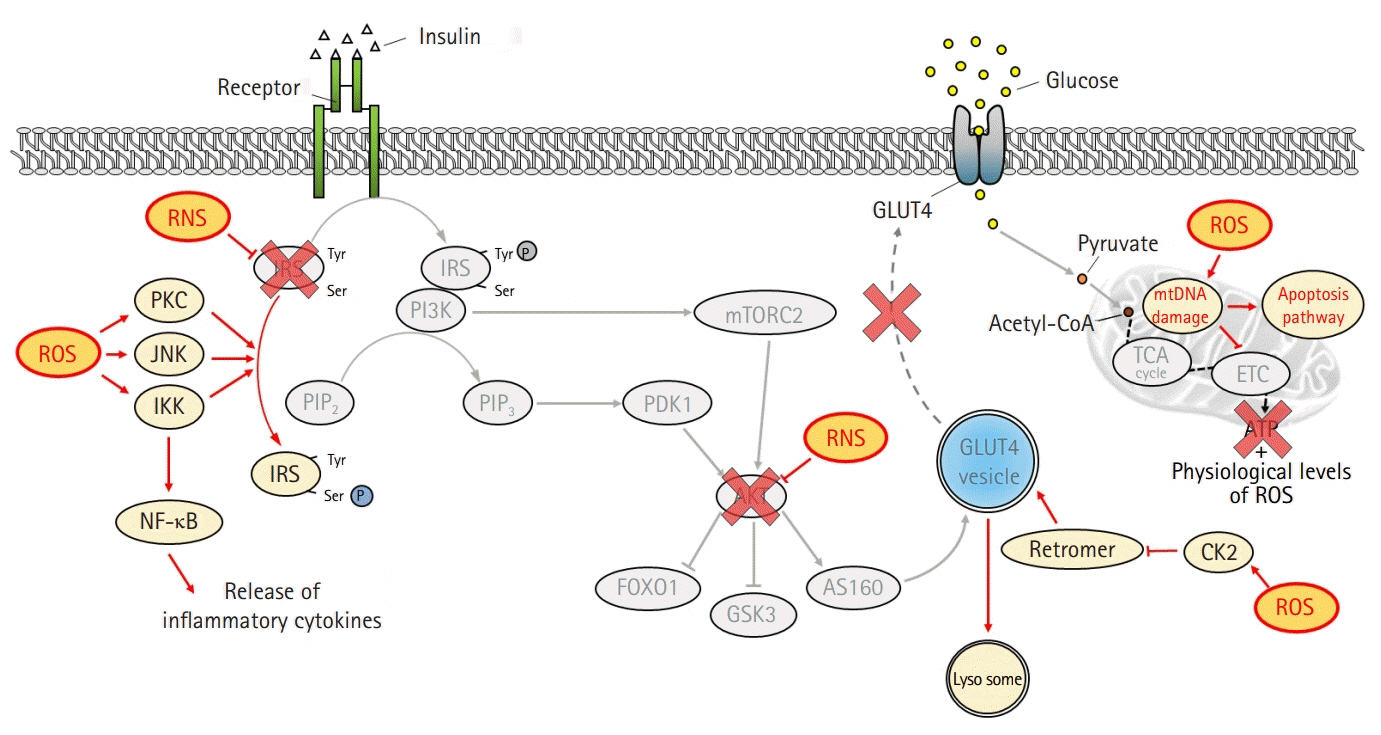
- The global obesity epidemic and the growing elderly population largely contribute to the increasing incidence of type 2 diabetes. Insulin resistance acts as a critical link between the present obesity pandemic and type 2 diabetes. Naturally occurring reactive oxygen species (ROS) regulate intracellular signaling and are kept in balance by the antioxidant system. However, the imbalance between ROS production and antioxidant capacity causes ROS accumulation and induces oxidative stress. Oxidative stress interrupts insulin-mediated intracellular signaling pathways, as supported by studies involving genetic modification of antioxidant enzymes in experimental rodents. In addition, a close association between oxidative stress and insulin resistance has been reported in numerous human studies. However, the controversial results with the use of antioxidants in type 2 diabetes raise the question of whether oxidative stress plays a critical role in insulin resistance. In this review article, we discuss the relevance of oxidative stress to insulin resistance based on genetically modified animal models and human trials.
Figure
Reference
-
References
1. Abdali D, Samson SE, Grover AK. How effective are antioxidant supplements in obesity and diabetes? Med Princ Pract. 2015; 24:201–15.
Article2. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013; 93:137–88.
Article3. Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol. 2017; 233:R15–42.
Article4. Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001; 86:3574–8.
Article5. Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011; 50:567–75.
Article6. Li R, Jia Z, Trush MA. Defining ROS in biology and medicine. React Oxyg Species (Apex). 2016; 1:9–21.
Article7. He L, He T, Farrar S, Ji L, Liu T, Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem. 2017; 44:532–53.
Article8. Trujillo M, Ferrer-Sueta G, Radi R. Peroxynitrite detoxification and its biologic implications. Antioxid Redox Signal. 2008; 10:1607–20.
Article9. Cheung PY, Wang W, Schulz R. Glutathione protects against myocardial ischemia-reperfusion injury by detoxifying peroxynitrite. J Mol Cell Cardiol. 2000; 32:1669–78.
Article10. Herrera R, Rosen OM. Autophosphorylation of the insulin receptor in vitro. Designation of phosphorylation sites and correlation with receptor kinase activation. J Biol Chem. 1986; 261:11980–5.
Article11. Sun XJ, Rothenberg P, Kahn CR, Backer JM, Araki E, Wilden PA, et al. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature. 1991; 352:73–7.
Article12. Hanke S, Mann M. The phosphotyrosine interactome of the insulin receptor family and its substrates IRS-1 and IRS-2. Mol Cell Proteomics. 2009; 8:519–34.
Article13. Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006; 3:343–53.14. Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997; 7:261–9.15. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005; 307:1098–101.
Article16. Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017; 169:381–405.
Article17. Imazu M, Strickland WG, Chrisman TD, Exton JH. Phosphorylation and inactivation of liver glycogen synthase by liver protein kinases. J Biol Chem. 1984; 259:1813–21.
Article18. Kwon HS, Huang B, Unterman TG, Harris RA. Protein kinase B-alpha inhibits human pyruvate dehydrogenase kinase-4 gene induction by dexamethasone through inactivation of FOXO transcription factors. Diabetes. 2004; 53:899–910.
Article19. Sano H, Kane S, Sano E, Mîinea CP, Asara JM, Lane WS, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003; 278:14599–602.
Article20. Cerf ME. Beta cell dysfunction and insulin resistance. Front Endocrinol (Lausanne). 2013; 4:37.
Article21. Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, et al. Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab. 2004; 287:E357–46.
Article22. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999; 48:1836–41.
Article23. Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002; 51:2005–11.24. Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011; 121:1858–70.
Article25. Oakes ND, Bell KS, Furler SM, Camilleri S, Saha AK, Ruderman NB, et al. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes. 1997; 46:2022–8.
Article26. Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005; 11:191–8.
Article27. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996; 271:665–8.
Article28. Barazzoni R, Zanetti M, Gortan Cappellari G, Semolic A, Boschelle M, Codarin E, et al. Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-κB inhibitor (IκB)-nuclear factor-κB (NFκB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia. 2012; 55:773–82.
Article29. Satapati S, Kucejova B, Duarte JA, Fletcher JA, Reynolds L, Sunny NE, et al. Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015; 125:4447–62.
Article30. Paglialunga S, Ludzki A, Root-McCaig J, Holloway GP. In adipose tissue, increased mitochondrial emission of reactive oxygen species is important for short-term high-fat diet-induced insulin resistance in mice. Diabetologia. 2015; 58:1071–80.
Article31. Chen XH, Zhao YP, Xue M, Ji CB, Gao CL, Zhu JG, et al. TNF-alpha induces mitochondrial dysfunction in 3T3-L1 adipocytes. Mol Cell Endocrinol. 2010; 328:63–9.32. Imoto K, Kukidome D, Nishikawa T, Matsuhisa T, Sonoda K, Fujisawa K, et al. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes. 2006; 55:1197–204.
Article33. Ma J, Nakagawa Y, Kojima I, Shibata H. Prolonged insulin stimulation down-regulates GLUT4 through oxidative stress-mediated retromer inhibition by a protein kinase CK2-dependent mechanism in 3T3-L1 adipocytes. J Biol Chem. 2014; 289:133–42.
Article34. Nomiyama T, Igarashi Y, Taka H, Mineki R, Uchida T, Ogihara T, et al. Reduction of insulin-stimulated glucose uptake by peroxynitrite is concurrent with tyrosine nitration of insulin receptor substrate-1. Biochem Biophys Res Commun. 2004; 320:639–47.
Article35. Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt- and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002; 277:32552–7.
Article36. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest. 2008; 118:789–800.
Article37. Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, et al. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One. 2010; 5:e11468.
Article38. Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017; 1863:1066–77.
Article39. Shin MG, Cha HN, Park S, Kim YW, Kim JY, Park SY. Selenoprotein W deficiency does not affect oxidative stress and insulin sensitivity in the skeletal muscle of high-fat diet-fed obese mice. Am J Physiol Cell Physiol. 2019; 317:C1172–82.
Article40. Heo JY, Cha HN, Kim KY, Lee E, Kim SJ, Kim YW, et al. Methionine sulfoxide reductase B1 deficiency does not increase high-fat diet-induced insulin resistance in mice. Free Radic Res. 2017; 51:24–37.
Article41. Muscogiuri G, Salmon AB, Aguayo-Mazzucato C, Li M, Balas B, Guardado-Mendoza R, et al. Genetic disruption of SOD1 gene causes glucose intolerance and impairs β-cell function. Diabetes. 2013; 62:4201–7.
Article42. Liu Y, Qi W, Richardson A, Van Remmen H, Ikeno Y, Salmon AB. Oxidative damage associated with obesity is prevented by overexpression of CuZn- or Mn-superoxide dismutase. Biochem Biophys Res Commun. 2013; 438:78–83.
Article43. Kang L, Dai C, Lustig ME, Bonner JS, Mayes WH, Mokshagundam S, et al. Heterozygous SOD2 deletion impairs glucose-stimulated insulin secretion, but not insulin action, in high-fat-fed mice. Diabetes. 2014; 63:3699–710.
Article44. Lark DS, Kang L, Lustig ME, Bonner JS, James FD, Neufer PD, et al. Enhanced mitochondrial superoxide scavenging does not improve muscle insulin action in the high fat-fed mouse. PLoS One. 2015; 10:e0126732.
Article45. Boden MJ, Brandon AE, Tid-Ang JD, Preston E, Wilks D, Stuart E, et al. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2012; 303:E798–805.
Article46. Park YS, Uddin MJ, Piao L, Hwang I, Lee JH, Ha H. Novel role of endogenous catalase in macrophage polarization in adipose tissue. Mediators Inflamm. 2016; 2016:8675905.
Article47. Piao L, Dorotea D, Jiang S, Koh EH, Oh GT, Ha H. Impaired peroxisomal fitness in obese mice, a vicious cycle exacerbating adipocyte dysfunction via oxidative stress. Antioxid Redox Signal. 2019; 31:1339–51.48. Amos DL, Robinson T, Massie MB, Cook C, Hoffsted A, Crain C, et al. Catalase overexpression modulates metabolic parameters in a new ‘stress-less’ leptin-deficient mouse model. Biochim Biophys Acta Mol Basis Dis. 2017; 1863:2293–306.
Article49. Kang L, Lustig ME, Bonner JS, Lee-Young RS, Mayes WH, James FD, et al. Mitochondrial antioxidative capacity regulates muscle glucose uptake in the conscious mouse: effect of exercise and diet. J Appl Physiol (1985). 2012; 113:1173–83.
Article50. Lee HY, Lee JS, Alves T, Ladiges W, Rabinovitch PS, Jurczak MJ, et al. Mitochondrial-targeted catalase protects against high-fat diet-induced muscle insulin resistance by decreasing intramuscular lipid accumulation. Diabetes. 2017; 66:2072–81.
Article51. McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004; 101:8852–7.
Article52. Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009; 10:260–72.
Article53. Merry TL, Tran M, Dodd GT, Mangiafico SP, Wiede F, Kaur S, et al. Hepatocyte glutathione peroxidase-1 deficiency improves hepatic glucose metabolism and decreases steatohepatitis in mice. Diabetologia. 2016; 59:2632–44.
Article54. Kim HR, Choi EJ, Kie JH, Lee JH, Seoh JY. Deficiency of glutathione peroxidase-1 and catalase attenuated diet-induced obesity and associated metabolic disorders. Acta Diabetol. 2020; 57:151–61.
Article55. Wohua Z, Weiming X. Glutaredoxin 2 (GRX2) deficiency exacerbates high fat diet (HFD)-induced insulin resistance, inflammation and mitochondrial dysfunction in brain injury: a mechanism involving GSK-3β. Biomed Pharmacother. 2019; 118:108940.
Article56. Cha HN, Park S, Dan Y, Kim JR, Park SY. Peroxiredoxin2 deficiency aggravates aging-induced insulin resistance and declines muscle strength. J Gerontol A Biol Sci Med Sci. 2019; 74:147–54.
Article57. Kim JH, Park SJ, Chae U, Seong J, Lee HS, Lee SR, et al. Peroxiredoxin 2 mediates insulin sensitivity of skeletal muscles through regulation of protein tyrosine phosphatase oxidation. Int J Biochem Cell Biol. 2018; 99:80–90.
Article58. Kim JH, Cha HN, Kim YW, Park SY. Peroxiredoxin 2 deficiency does not affect insulin resistance and oxidative stress in high-fat diet-fed obese mice. Arch Physiol Biochem. 2020; Mar. 6. [Epub]. Arch Physiol Biochem https://doi.org/10.1080/13813455.2020.1733026.
Article59. Huh JY, Kim Y, Jeong J, Park J, Kim I, Huh KH, et al. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxid Redox Signal. 2012; 16:229–43.
Article60. Chen L, Na R, Gu M, Salmon AB, Liu Y, Liang H, et al. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell. 2008; 7:866–78.61. Nabeshima A, Yamada S, Guo X, Tanimoto A, Wang KY, Shimajiri S, et al. Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxid Redox Signal. 2013; 19:1983–98.
Article62. Pacifici F, Arriga R, Sorice GP, Capuani B, Scioli MG, Pastore D, et al. Peroxiredoxin 6, a novel player in the pathogenesis of diabetes. Diabetes. 2014; 63:3210–20.
Article63. Styskal J, Nwagwu FA, Watkins YN, Liang H, Richardson A, Musi N, et al. Methionine sulfoxide reductase A affects insulin resistance by protecting insulin receptor function. Free Radic Biol Med. 2013; 56:123–32.64. Hunnicut J, Liu Y, Richardson A, Salmon AB. MsrA overexpression targeted to the mitochondria, but not cytosol, preserves insulin sensitivity in diet-induced obese mice. PLoS One. 2015; 10:e0139844.
Article65. Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007; 9:343–53.
Article66. Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, et al. Protein kinase C theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J Biol Chem. 2004; 279:45304–7.67. Ju TJ, Kwon WY, Kim YW, Kim JY, Kim YD, Lee IK, et al. Hemin improves insulin sensitivity in skeletal muscle in high fat-fed mice. J Pharmacol Sci. 2014; 126:115–25.
Article68. Kim BS, Cha HN, Kim YW, Kim JY, Dan JM, Park SY. Inhibition of lipid infusion-induced skeletal muscle insulin resistance by cotreatment with tempol and glutathione in mice. J Pharmacol Sci. 2009; 110:370–80.
Article69. Gao M, Zhao Z, Lv P, Li Y, Gao J, Zhang M, et al. Quantitative combination of natural anti-oxidants prevents metabolic syndrome by reducing oxidative stress. Redox Biol. 2015; 6:206–17.
Article70. Lebel M, Massip L, Garand C, Thorin E. Ascorbate improves metabolic abnormalities in Wrn mutant mice but not the free radical scavenger catechin. Ann N Y Acad Sci. 2010; 1197:40–4.
Article71. Telci A, Cakatay U, Kayali R, Erdoğan C, Orhan Y, Sivas A, et al. Oxidative protein damage in plasma of type 2 diabetic patients. Horm Metab Res. 2000; 32:40–3.
Article72. Piwowar A, Knapik-Kordecka M, Warwas M. Markers of oxidative protein damage in plasma and urine of type 2 diabetic patients. Br J Biomed Sci. 2009; 66:194–9.
Article73. Atli T, Keven K, Avci A, Kutlay S, Turkcapar N, Varli M, et al. Oxidative stress and antioxidant status in elderly diabetes mellitus and glucose intolerance patients. Arch Gerontol Geriatr. 2004; 39:269–75.
Article74. Song F, Jia W, Yao Y, Hu Y, Lei L, Lin J, et al. Oxidative stress, antioxidant status and DNA damage in patients with impaired glucose regulation and newly diagnosed type 2 diabetes. Clin Sci (Lond). 2007; 112:599–606.
Article75. Konukoglu D, Turhan MS, Ercan M, Serin O. Relationship between plasma leptin and zinc levels and the effect of insulin and oxidative stress on leptin levels in obese diabetic patients. J Nutr Biochem. 2004; 15:757–60.
Article76. Ceriello A, Falleti E, Bortolotti N, Motz E, Cavarape A, Russo A, et al. Increased circulating intercellular adhesion molecule-1 levels in type II diabetic patients: the possible role of metabolic control and oxidative stress. Metabolism. 1996; 45:498–501.
Article77. Dong QY, Cui Y, Chen L, Song J, Sun L. Urinary 8-hydroxydeoxyguanosine levels in diabetic retinopathy patients. Eur J Ophthalmol. 2008; 18:94–8.
Article78. Cakatay U. Protein oxidation parameters in type 2 diabetic patients with good and poor glycaemic control. Diabetes Metab. 2005; 31:551–7.
Article79. Cominacini L, Fratta Pasini A, Garbin U, Campagnola M, Davoli A, Rigoni A, et al. E-selectin plasma concentration is influenced by glycaemic control in NIDDM patients: possible role of oxidative stress. Diabetologia. 1997; 40:584–9.
Article80. Suzuki S, Hinokio Y, Komatu K, Ohtomo M, Onoda M, Hirai S, et al. Oxidative damage to mitochondrial DNA and its relationship to diabetic complications. Diabetes Res Clin Pract. 1999; 45:161–8.
Article81. Ingram KH, Hill H, Moellering DR, Hill BG, Lara-Castro C, Newcomer B, et al. Skeletal muscle lipid peroxidation and insulin resistance in humans. J Clin Endocrinol Metab. 2012; 97:E1182–6.
Article82. Torres SH, De Sanctis JB, de L Briceño M, Hernández N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol. 2004; 181:419–27.
Article83. Akkuş I, Kalak S, Vural H, Caglayan O, Menekşe E, Can G, et al. Leukocyte lipid peroxidation, superoxide dismutase, glutathione peroxidase and serum and leukocyte vitamin C levels of patients with type II diabetes mellitus. Clin Chim Acta. 1996; 244:221–7.
Article84. Lin H, Ye S, Xu J, Wang W. The alpha-lipoic acid decreases urinary podocalyxin excretion in type 2 diabetics by inhibiting oxidative stress in vivo. J Diabetes Complications. 2015; 29:64–7.
Article85. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–61.
Article86. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009; 119:573–81.
Article87. Konopka AR, Asante A, Lanza IR, Robinson MM, Johnson ML, Dalla Man C, et al. Defects in mitochondrial efficiency and H2O2 emissions in obese women are restored to a lean phenotype with aerobic exercise training. Diabetes. 2015; 64:2104–15.
Article88. Tushuizen ME, Nieuwland R, Scheffer PG, Sturk A, Heine RJ, Diamant M. Two consecutive high-fat meals affect endothelial-dependent vasodilation, oxidative stress and cellular microparticles in healthy men. J Thromb Haemost. 2006; 4:1003–10.
Article89. Samocha-Bonet D, Campbell LV, Mori TA, Croft KD, Greenfield JR, Turner N, et al. Overfeeding reduces insulin sensitivity and increases oxidative stress, without altering markers of mitochondrial content and function in humans. PLoS One. 2012; 7:e36320.
Article90. Reitman A, Friedrich I, Ben-Amotz A, Levy Y. Low plasma antioxidants and normal plasma B vitamins and homocysteine in patients with severe obesity. Isr Med Assoc J. 2002; 4:590–3.91. Hasegawa G, Yamamoto Y, Zhi JG, Tanino Y, Yamasaki M, Yano M, et al. Daily profile of plasma %CoQ10 level, a biomarker of oxidative stress, in patients with diabetes manifesting postprandial hyperglycaemia. Acta Diabetol. 2005; 42:179–81.
Article92. van der Schaft N, Schoufour JD, Nano J, Kiefte-de Jong JC, Muka T, Sijbrands EJ, et al. Dietary antioxidant capacity and risk of type 2 diabetes mellitus, prediabetes and insulin resistance: the Rotterdam Study. Eur J Epidemiol. 2019; 34:853–61.
Article93. Mancini FR, Affret A, Dow C, Balkau B, Bonnet F, Boutron-Ruault MC, et al. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia. 2018; 61:308–16.
Article94. Kataja-Tuomola MK, Kontto JP, Männistö S, Albanes D, Virtamo JR. Effect of alpha-tocopherol and beta-carotene supplementation on macrovascular complications and total mortality from diabetes: results of the ATBC Study. Ann Med. 2010; 42:178–86.
Article95. Sluijs I, Cadier E, Beulens JW, van der A DL, Spijkerman AM, van der Schouw YT. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr Metab Cardiovasc Dis. 2015; 25:376–81.
Article96. Eshak ES, Iso H, Muraki I, Tamakoshi A. Among the water-soluble vitamins, dietary intakes of vitamins C, B2 and folate are associated with the reduced risk of diabetes in Japanese women but not men. Br J Nutr. 2019; 121:1357–64.
Article97. Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. The effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018; 55:341–53.
Article98. Choi SW, Ho CK. Antioxidant properties of drugs used in type 2 diabetes management: could they contribute to, confound or conceal effects of antioxidant therapy? Redox Rep. 2018; 23:1–24.
Article99. Dao VT, Casas AI, Maghzal GJ, Seredenina T, Kaludercic N, Robledinos-Anton N, et al. Pharmacology and clinical drug candidates in redox medicine. Antioxid Redox Signal. 2015; 23:1113–29.
Article100. Bonetta R. Potential therapeutic applications of MnSODs and SOD-mimetics. Chemistry. 2018; 24:5032–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Colored Foods and Diabetes
- Metabolism-Centric Overview of the Pathogenesis of Alzheimer's Disease
- Effects of Antioxidant Vitamins and Magnesium Supplementation on Fasting Blood Glucose and Lipids in Patients with Type 2 Diabetes
- Intake of Fruits for Diabetics: Why and How Much?
- Metabolic Syndrome and Oxidative Stress, Antioxidants