Ann Dermatol.
2012 Nov;24(4):398-405. 10.5021/ad.2012.24.4.398.
Interleukin-17 and Interleukin-22 Induced Proinflammatory Cytokine Production in Keratinocytes via Inhibitor of Nuclear Factor kappaB Kinase-alpha Expression
- Affiliations
-
- 1Department of Microbiology, School of Medicine, Ewha Womans University, Seoul, Korea.
- 2Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea. beauty4u@catholic.ac.kr
- 3Department of Psychiatry, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2266033
- DOI: http://doi.org/10.5021/ad.2012.24.4.398
Abstract
- BACKGROUND
The pathogenesis of psoriasis may involve the interleukin (IL)-23 and Th17-mediated immune responses. Th17 cells secret IL-17 and IL-22, which mediates dermal inflammation and acanthosis.
OBJECTIVE
As inhibitor of nuclear factor kappaB kinase-alpha (IKKalpha) has been previously identified as a primary regulator of keratinocyte differentiation and proliferation, we proposed that IL-17 and IL-22 might affect keratinocyte differentiation by changing the expression of IKKalpha.
METHODS
We employed HaCaT cells maintained culture medium at a low calcium concentration (0.06 mM) and induced differentiation by switching to the high concentration (2.8 mM) media with IL-17 or IL-22, then compared the IKKalpha expression and the cell cycle. We employed reconstituted human epidermal skin (Neoderm) and mice ears for the in vivo studies.
RESULTS
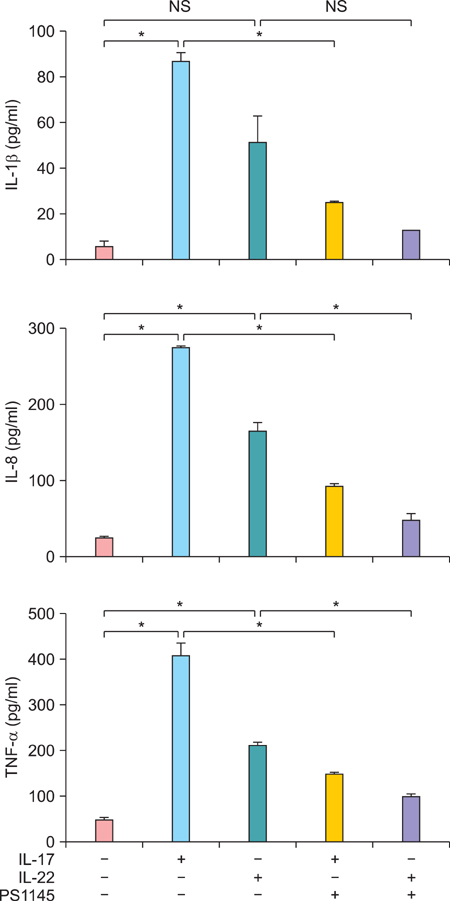
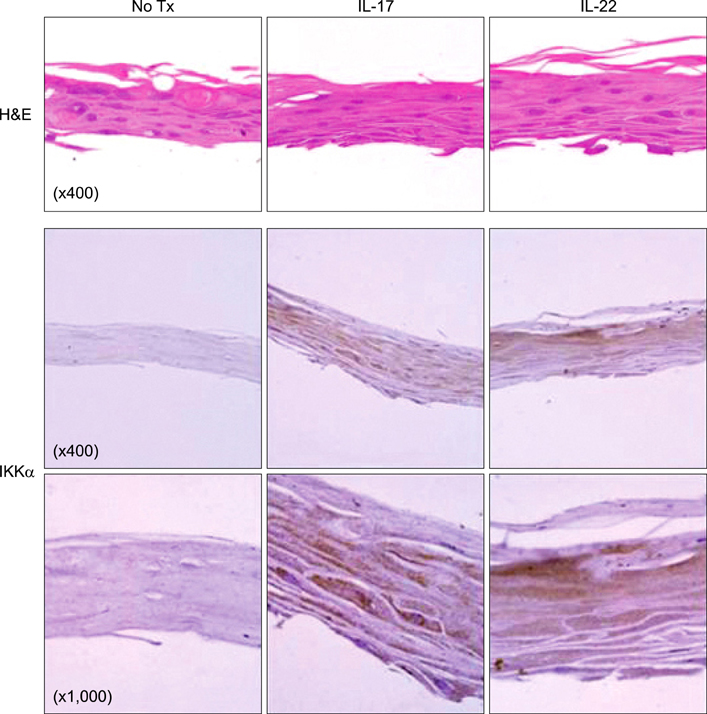
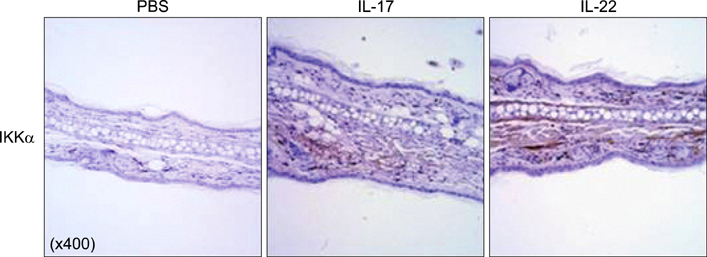
Elevated calcium concentration induced IKKalpha expression and terminal differentiation with cell cycle arrest in HaCaT cell cultures. Moreover, IL-17 and IL-22 treatment also induced IKKalpha in HaCaT cells and reconstituted human epidermis. IKKalpha induction was also noted, following the injection of IL-17 and IL-22 into mice ears.
CONCLUSION
Although the induction of IKKalpha was accompanied by keratinocyte differentiation, IL-17 and IL-22 did not affect calcium-mediated differentiation or the cell cycle. Rather, IL-17 and IL-22 appear to contribute to the inflammation occurring via the induction of IKKalpha from keratinocytes or skin layers.
Keyword
MeSH Terms
Figure
Reference
-
1. Tonel G, Conrad C. Interplay between keratinocytes and immune cells--recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009. 41:963–968.
Article2. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008. 118:597–607.
Article3. Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008. 159:1092–1102.
Article4. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007. 445:648–651.
Article5. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005. 174:3695–3702.
Article6. Mauro T, Bench G, Sidderas-Haddad E, Feingold K, Elias P, Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J Invest Dermatol. 1998. 111:1198–1201.
Article7. Elias PM, Ahn SK, Denda M, Brown BE, Crumrine D, Kimutai LK, et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002. 119:1128–1136.
Article8. Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001. 410:710–714.
Article9. Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004. 428:660–664.
Article10. Deyrieux AF, Wilson VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007. 54:77–83.
Article11. Fuchs E, Byrne C. The epidermis: rising to the surface. Curr Opin Genet Dev. 1994. 4:725–736.
Article12. Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002. 277:16639–16647.13. Liu B, Zhu F, Xia X, Park E, Hu Y. A tale of terminal differentiation: IKKalpha, the master keratinocyte regulator. Cell Cycle. 2009. 8:527–531.
Article14. Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, et al. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009. 182:1631–1640.
Article15. Leis H, Sanchis A, Pérez P. Deletion of the N-terminus of IKKgamma induces apoptosis in keratinocytes and impairs the AKT/PTEN signaling pathway. Exp Cell Res. 2007. 313:742–752.
Article16. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004. 21:241–254.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Interleukin-17 Production in Patients with Rheumatoid Arthritis by Phosphoinositide 3-kinase (PI3K)/ Akt and Nuclear Factor KappaB (NF-kappaB) Dependent Signal Transduction Pathway
- Blockade of p38 Mitogen-activated Protein Kinase Pathway Inhibits Interleukin-6 Release and Expression in Primary Neonatal Cardiomyocytes
- Induction of Interleukin-22 (IL-22) production in CD4+ T Cells by IL-17A Secreted from CpG-Stimulated Keratinocytes
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Pyrrolidine Dithiocarbamate Inhibits Nuclear Factor kappaB and Toll-Like Receptor 4 Expression in Rats with Acute Necrotizing Pancreatitis