Allergy Asthma Immunol Res.
2010 Apr;2(2):65-76. 10.4168/aair.2010.2.2.65.
The Pathophysiology, Diagnosis and Treatment of Allergic Rhinitis
- Affiliations
-
- 1Department of Otorhinolaryngology, Seoul National University College of Medicine, Seoul, Korea. ygmin312@dreamwiz.com
- KMID: 2260592
- DOI: http://doi.org/10.4168/aair.2010.2.2.65
Abstract
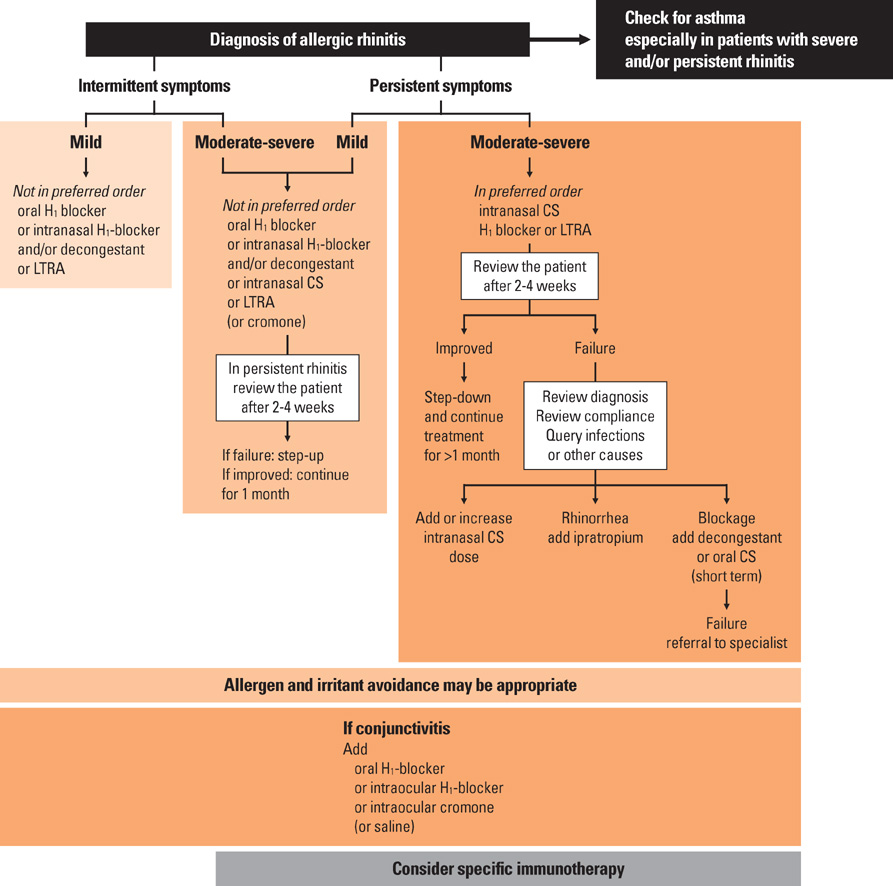
- Treatment of AR requires a stepwise approach depending on the severity and duration of symptoms. Treatment options for AR consist of allergen avoidance, pharmacotherapy, immunotherapy and surgery. For the mechanisms of AR, anti-IgE antibody and specific antibody to cytokines such as IL-4 or IL-5 that correlate with allergic inflammation have recently emerged. SLIT is currently widely used due to its efficacy, safety and convenience, which replaces subcutaneous immunotherapy. Although allergen avoidance and immunotherapy are theoretically ideal, antihistamines and intranasal corticosteroids will play the main role in the management of AR until an innovative treatment develops. However, patients' main symptom, the duration and severity of AR, patients' compliance, safety of medication and cost-effectiveness should be considered when treatment options are chosen. In conclusion, physicians should be aware of etiology, pathophysiology, symptoms, signs and diseases related to AR in order to make a correct diagnosis and choose a proper treatment option for each patient.
Keyword
MeSH Terms
-
Adrenal Cortex Hormones
Antibodies, Anti-Idiotypic
Compliance
Cytokines
Histamine Antagonists
Humans
Hypersensitivity
Immunotherapy
Inflammation
Interleukin-4
Interleukin-5
Rhinitis
Rhinitis, Allergic, Perennial
Rhinitis, Allergic, Seasonal
Adrenal Cortex Hormones
Antibodies, Anti-Idiotypic
Cytokines
Histamine Antagonists
Interleukin-4
Interleukin-5
Figure
Cited by 4 articles
-
Diagnosis and treatment of allergic rhinitis
Young Hoon Kim, Kyung-Su Kim
J Korean Med Assoc. 2010;53(9):780-790. doi: 10.5124/jkma.2010.53.9.780.Climate change and respiratory allergic diseases
Sang-Heon Kim, Ho Joo Yoon
J Korean Med Assoc. 2011;54(2):161-168. doi: 10.5124/jkma.2011.54.2.161.The correlation between allergy sensitization rate in pediatric and aerobiological study for airborne pollen in Busan for 15 years
Myong Soon Sung, Yong-Jin Park, Geun Hwa Park, Jae Won Oh, Sung Won Kim
Allergy Asthma Respir Dis. 2014;2(1):38-47. doi: 10.4168/aard.2014.2.1.38.Ocular allergy in the Asia Pacific region
Constance H. Katelaris
Asia Pac Allergy. 2011;1(3):108-114. doi: 10.5415/apallergy.2011.1.3.108.
Reference
-
1. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001. 108:S147–S334.2. Nathan RA. The burden of allergic rhinitis. Allergy Asthma Proc. 2007. 28:3–9.3. Berger WE. Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs. 2004. 6:233–250.4. Settipane RA. Rhinitis: a dose of epidemiological reality. Allergy Asthma Proc. 2003. 24:147–154.5. Min YG, Choi BY, Kwon SK, Lee SS, Jung YH, Kim JW, Oh SJ. Multicenter study on the prevalence of perennial allergic rhinitis and allergy-associated disorders. J Korean Med Sci. 2001. 16:697–701.6. Lee SI, Shin MH, Lee HB, Lee JS, Son BK, Koh YY, Kim KE, Ahn YO. Prevalences of symptoms of asthma and other allergic diseases in korean children: a nationwide questionnaire survey. J Korean Med Sci. 2001. 16:155–164.7. Reed SD, Lee TA, McCrory DC. The economic burden of allergic rhinitis: a critical evaluation of the literature. Pharmacoeconomics. 2004. 22:345–361.8. Nash DB, Sullivan SD, Mackowiak J. Optimizing quality of care and cost effectiveness in treating allergic rhinitis in a managed care setting. Am J Manag Care. 2000. 6:S3–S15. quiz S19-20.9. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Ait-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D. World Health Organization. GA(2)LEN. AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allerg. 2008. 63:Suppl 86. 8–160.10. Chaplin DD. 1. Overview of the human immune response. J Allergy Clin Immunol. 2006. 117:S430–S435.11. Broide DH. The pathophysiology of allergic rhinoconjunctivitis. Allergy Asthma Proc. 2007. 28:398–403.12. Prussin C, Metcalfe DD. 5. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2006. 117:S450–S456.13. Kay AB. Allergy and allergic diseases. Second of two parts. N Engl J Med. 2001. 344:109–113.14. Togias A. Unique mechanistic features of allergic rhinitis. J Allergy Clin Immunol. 2000. 105:S599–S604.15. Gerth van Wijk RG, de Graaf-in't Veld C, Garrelds IM. Nasal hyperreactivity. Rhinology. 1999. 37:50–55.16. Linneberg A, Henrik Nielsen N, Frolund L, Madsen F, Dirksen A, Jorgensen T. The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002. 57:1048–1052.17. Montnemery P, Svensson C, Adelroth E, Lofdahl CG, Andersson M, Greiff L, Persson CG. Prevalence of nasal symptoms and their relation to self-reported asthma and chronic bronchitis/emphysema. Eur Respir J. 2001. 17:596–603.18. Gaga M, Lambrou P, Papageorgiou N, Koulouris NG, Kosmas E, Fragakis S, Sofios C, Rasidakis A, Jordanoglou J. Eosinophils are a feature of upper and lower airway pathology in non-atopic asthma, irrespective of the presence of rhinitis. Clin Exp Allergy. 2000. 30:663–669.19. Bousquet J, Annesi-Maesano I, Carat F, Leger D, Rugina M, Pribil C, El Hasnaoui A, Chanal I. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy. 2005. 35:728–732.20. Leynaert B, Neukirch C, Kony S, Guenegou A, Bousquet J, Aubier M, Neukirch F. Association between asthma and rhinitis according to atopic sensitization in a population-based study. J Allergy Clin Immunol. 2004. 113:86–93.21. Downie SR, Andersson M, Rimmer J, Leuppi JD, Xuan W, Akerlund A, Peat JK, Salome CM. Association between nasal and bronchial symptoms in subjects with persistent allergic rhinitis. Allergy. 2004. 59:320–326.22. Chakir J, Laviolette M, Boutet M, Laliberte R, Dube J, Boulet LP. Lower airways remodeling in nonasthmatic subjects with allergic rhinitis. Lab Invest. 1996. 75:735–744.23. Djukanovic R, Lai CK, Wilson JW, Britten KM, Wilson SJ, Roche WR, Howarth PH, Holgate ST. Bronchial mucosal manifestations of atopy: a comparison of markers of inflammation between atopic asthmatics, atopic nonasthmatics and healthy controls. Eur Respir J. 1992. 5:538–544.24. Brown JL, Behndig AF, Sekerel BE, Pourazar J, Blomberg A, Kelly FJ, Sandstrom T, Frew AJ, Wilson SJ. Lower airways inflammation in allergic rhinitics: a comparison with asthmatics and normal controls. Clin Exp Allergy. 2007. 37:688–695.25. Marcucci F, Passalacqua G, Canonica GW, Frati F, Salvatori S, Di cara G, Petrini I, Bernini M, Novembre E, Bernardini R, Incorvaia C, Sensi LG. Lower airway inflammation before and after house dust mite nasal challenge: an age and allergen exposure-related phenomenon. Respir Med. 2007. 101:1600–1608.26. Madonini E, Briatico-Vangosa G, Pappacoda A, Maccagni G, Cardani A, Saporiti F. Seasonal increase of bronchial reactivity in allergic rhinitis. J Allergy Clin Immunol. 1987. 79:358–363.27. Corren J, Adinoff AD, Irvin CG. Changes in bronchial responsiveness following nasal provocation with allergen. J Allergy Clin Immunol. 1992. 89:611–618.28. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003. 111:1171–1183. quiz 1184.29. Igarashi Y, Goldrich MS, Kaliner MA, Irani AM, Schwartz LB, White MV. Quantitation of inflammatory cells in the nasal mucosa of patients with allergic rhinitis and normal subjects. J Allergy Clin Immunol. 1995. 95:716–725.30. Holgate ST, Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet. 2006. 368:780–793.31. Bang JH, Kim YJ, Shin HS, Lee BJ. Cinical analysis of allergic rhinitis in Seoul. J Rhinol. 1996. 3:130–134.32. Finnerty JP, Summerell S, Holgate ST. Relationship between skin-prick tests, the multiple allergosorbent test and symptoms of allergic disease. Clin Exp Allergy. 1989. 19:51–56.33. Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002. 109:419–425.34. Silvestri M, Battistini E, Defilippi AC, Sabatini F, Sale R, Pecora S, Rossi GA. Early decrease in nasal eosinophil proportion after nasal allergen challenge correlates with baseline bronchial reactivity to methacholine in children sensitized to house dust mites. J Investig Allergol Clin Immunol. 2005. 15:266–276.35. Choi SH, Yoo Y, Yu J, Rhee CS, Min YG, Koh YY. Bronchial hyperresponsiveness in young children with allergic rhinitis and its risk factors. Allergy. 2007. 62:1051–1056.36. Ferdousi HA, Zetterstrom O, Dreborg S. Bronchial hyper-responsiveness predicts the development of mild clinical asthma within 2 yr in school children with hay-fever. Pediatr Allergy Immunol. 2005. 16:478–486.37. Choi SY, Lee IY, Shon JH, Lee YW, Shin SH, Lee DH, Kim PH, Yong TS, Hong CS, Park JW. Effect of steam-drum laundry machine for removal of allergens. J Asthma Allergy Clin Immunol. 2006. 26:289–296. Korean.38. Moscato G, Vandenplas O, Van Wijk RG, Malo JL, Perfetti L, Quirce S, Walusiak J, Castano R, Pala G, Gautrin D, De Groot H, Folletti I, Yacoub MR, Siracusa A. European Academy of Allergology and Clinical Immunolgy. EAACI position paper on occupational rhinitis. Respir Res. 2009. 10:16.39. Bousquet J, Van Cauwenberge P, Bachert C, Canonica GW, Demoly P, Durham SR, Fokkens W, Lockey R, Meltzer EO, Mullol J, Naclerio RM, Price D, Simons FE, Vignola AM, Warner JO. Requirements for medications commonly used in the treatment of allergic rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and its Impact on Asthma (ARIA). Allergy. 2003. 58:192–197.40. Simons FE. Advances in H1-antihistamines. N Engl J Med. 2004. 351:2203–2217.41. de Blic J, Wahn U, Billard E, Alt R, Pujazon MC. Levocetirizine in children: evidenced efficacy and safety in a 6-week randomized seasonal allergic rhinitis trial. Pediatr Allergy Immunol. 2005. 16:267–275.42. McNeely W, Wiseman LR. Intranasal azelastine. A review of its efficacy in the management of allergic rhinitis. Drugs. 1998. 56:91–114.43. Yanez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002. 89:479–484.44. Berger WE, White MV. Efficacy of azelastine nasal spray in patients with an unsatisfactory response to loratadine. Ann Allergy Asthma Immunol. 2003. 91:205–211.45. Lee BJ, Kim YJ, Kim JH, Shin HS, Chung YS. A comparative study of intranasal budesonide and oral terfenadine in perennial allergic rhinitics: effect on the symptom score and nasal secretion eosinophils. J Asthma Allergy Clin Immunol. 2001. 21:216–222. Korean.46. Bhatia S, Baroody FM, deTineo M, Naclerio RM. Increased nasal airflow with budesonide compared with desloratadine during the allergy season. Arch Otolaryngol Head Neck Surg. 2005. 131:223–228.47. Selner JC, Weber RW, Richmond GW, Stricker WE, Norton JD. Onset of action of aqueous beclomethasone dipropionate nasal spray in seasonal allergic rhinitis. Clin Ther. 1995. 17:1099–1109.48. Skoner DP, Rachelefsky GS, Meltzer EO, Chervinsky P, Morris RM, Seltzer JM, Storms WW, Wood RA. Detection of growth suppression in children during treatment with intranasal beclomethasone dipropionate. Pediatrics. 2000. 105:E23.49. Schenkel EJ, Skoner DP, Bronsky EA, Miller SD, Pearlman DS, Rooklin A, Rosen JP, Ruff ME, Vandewalker ML, Wanderer A, Damaraju CV, Nolop KB, Mesarina-Wicki B. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray. Pediatrics. 2000. 105:E22.50. Allen DB, Meltzer EO, Lemanske RF Jr, Philpot EE, Faris MA, Kral KM, Prillaman BA, Rickard KA. No growth suppression in children treated with the maximum recommended dose of fluticasone propionate aqueous nasal spray for one year. Allergy Asthma Proc. 2002. 23:407–413.51. Watson WT, Becker AB, Simons FE. Treatment of allergic rhinitis with intranasal corticosteroids in patients with mild asthma: effect on lower airway responsiveness. J Allergy Clin Immunol. 1993. 91:97–101.52. Foresi A, Pelucchi A, Gherson G, Mastropasqua B, Chiapparino A, Testi R. Once daily intranasal fluticasone propionate (200 micrograms) reduces nasal symptoms and inflammation but also attenuates the increase in bronchial responsiveness during the pollen season in allergic rhinitis. J Allergy Clin Immunol. 1996. 98:274–282.53. Meltzer EO, Malmstrom K, Lu S, Prenner BM, Wei LX, Weinstein SF, Wolfe JD, Reiss TF. Concomitant montelukast and loratadine as treatment for seasonal allergic rhinitis: a randomized, placebo-controlled clinical trial. J Allergy Clin Immunol. 2000. 105:917–922.54. Nayak AS, Philip G, Lu S, Malice MP, Reiss TF. Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002. 88:592–600.55. Kurowski M, Kuna P, Gorski P. Montelukast plus cetirizine in the prophylactic treatment of seasonal allergic rhinitis: influence on clinical symptoms and nasal allergic inflammation. Allergy. 2004. 59:280–288.56. Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005. 115:459–465.57. Plewako H, Arvidsson M, Petruson K, Oancea I, Holmberg K, Adelroth E, Gustafsson H, Sandstrom T, Rak S. The effect of omalizumab on nasal allergic inflammation. J Allergy Clin Immunol. 2002. 110:68–71.58. Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol. 2004. 114:527–530.59. Casale TB, Busse WW, Kline JN, Ballas ZK, Moss MH, Townley RG, Mokhtarani M, Seyfert-Margolis V, Asare A, Bateman K, Deniz Y. Omalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitis. J Allergy Clin Immunol. 2006. 117:134–140.60. Price KS, Hamilton RG. Anaphylactoid reactions in two patients after omalizumab administration after successful long-term therapy. Allergy Asthma Proc. 2007. 28:313–319.61. Cohen SG, Evans R 3rd. Allergen immunotherapy in historical perspective. Clin Allergy Immunol. 2004. 18:1–36.62. Chang H, Han DH, Mo JH, Kim JW, Kim DY, Lee CH, Min YG, Rhee CS. Early compliance and efficacy of sublingual immunotherapy in patients with allergic rhinitis for house dust mites. Clin Exp Otorhinolaryngol. 2009. 2:136–140.63. Potter PC. Update on sublingual immunotherapy. Ann Allergy Asthma Immunol. 2006. 96:S22–S25.64. Frew AJ. Sublingual immunotherapy. N Engl J Med. 2008. 358:2259–2264.65. Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005. 60:4–12.66. Pajno GB, Morabito L, Barberio G, Parmiani S. Clinical and immunologic effects of long-term sublingual immunotherapy in asthmatic children sensitized to mites: a double-blind, placebo-controlled study. Allergy. 2000. 55:842–849.67. Penagos M, Compalati E, Tarantini F, Baena-Cagnani R, Huerta J, Passalacqua G, Canonica GW. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol. 2006. 97:141–148.68. Novembre E, Galli E, Landi F, Caffarelli C, Pifferi M, De Marco E, Burastero SE, Calori G, Benetti L, Bonazza P, Puccinelli P, Parmiani S, Bernardini R, Vierucci A. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004. 114:851–857.69. Di Rienzo V, Marcucci F, Puccinelli P, Parmiani S, Frati F, Sensi L, Canonica GW, Passalacqua G. Long-lasting effect of sublingual immunotherapy in children with asthma due to house dust mite: a 10-year prospective study. Clin Exp Allergy. 2003. 33:206–210.70. Marogna M, Tomassetti D, Bernasconi A, Colombo F, Massolo A, Businco AD, Canonica GW, Passalacqua G, Tripodi S. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008. 101:206–211.71. Dunsky EH, Goldstein MF, Dvorin DJ, Belecanech GA. Anaphylaxis to sublingual immunotherapy. Allergy. 2006. 61:1235.72. Antico A, Pagani M, Crema A. Anaphylaxis by latex sublingual immunotherapy. Allergy. 2006. 61:1236–1237.73. Eifan AO, Keles S, Bahceciler NN, Barlan IB. Anaphylaxis to multiple pollen allergen sublingual immunotherapy. Allergy. 2007. 62:567–568.74. Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006. 117:1021–1035.75. Naclerio RM, deTineo ML, Baroody FM. Ragweed allergic rhinitis and the paranasal sinuses. A computed tomographic study. Arch Otolaryngol Head Neck Surg. 1997. 123:193–196.76. Piette V, Bousquet C, Kvedariene V, Dhivert-Donnadieu H, Crampette L, Senac JP, Bousquet J, Demoly P. Sinus CT scans and mediator release in nasal secretions after nasal challenge with cypress pollens. Allergy. 2004. 59:863–868.77. Braun JJ, Alabert JP, Michel FB, Quiniou M, Rat C, Cougnard J, Czarlewski W, Bousquet J. Adjunct effect of loratadine in the treatment of acute sinusitis in patients with allergic rhinitis. Allergy. 1997. 52:650–655.78. Min YG, Kim HS, Suh SH, Jeon SY, Son YI, Yoon S. Paranasal sinusitis after long-term use of topical nasal decongestants. Acta Otolaryngol. 1996. 116:465–471.79. Rhee CS, Min YG, Lee CH. Expression of IL-4, IL-5 and IFN-gamma mRNAs in patients with nasal polyps. Korean J Otolaryngol-Head Neck Surg. 1996. 39:1243–1248.80. Alobid I, Benitez P, Valero A, Berenguer J, Bernal-Sprekelsen M, Picado C, Mullol J. The impact of atopy, sinus opacification, and nasal patency on quality of life in patients with severe nasal polyposis. Otolaryngol Head Neck Surg. 2006. 134:609–612.81. Vinke JG, KleinJan A, Severijnen LW, Hoeve LJ, Fokkens WJ. Differences in nasal cellular infiltrates between allergic children and age-matched controls. Eur Respir J. 1999. 13:797–803.82. Nguyen LH, Manoukian JJ, Sobol SE, Tewfik TL, Mazer BD, Schloss MD, Taha R, Hamid QA. Similar allergic inflammation in the middle ear and the upper airway: evidence linking otitis media with effusion to the united airways concept. J Allergy Clin Immunol. 2004. 114:1110–1115.83. Cassano P, Gelardi M, Cassano M, Fiorella ML, Fiorella R. Adenoid tissue rhinopharyngeal obstruction grading based on fiberendoscopic findings: a novel approach to therapeutic management. Int J Pediatr Otorhinolaryngol. 2003. 67:1303–1309.84. Georgalas C, Thomas K, Owens C, Abramovich S, Lack G. Medical treatment for rhinosinusitis associated with adenoidal hypertrophy in children: an evaluation of clinical response and changes on magnetic resonance imaging. Ann Otol Rhinol Laryngol. 2005. 114:638–644.85. Tewfik TL, Mazer B. The links between allergy and otitis media with effusion. Curr Opin Otolaryngol Head Neck Surg. 2006. 14:187–190.86. Marshall PS, O'Hara C, Steinberg P. Effects of seasonal allergic rhinitis on selected cognitive abilities. Ann Allergy Asthma Immunol. 2000. 84:403–410.87. Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007. 120:381–387.