Korean J Lab Med.
2006 Aug;26(4):287-293. 10.3343/kjlm.2006.26.4.287.
Modulation of Telomerase Activity and Human Telomerase Reverse Transcriptase Expression by Caspases and Bcl-2 Family Proteins in Cisplatin-Induced Cell Death
- Affiliations
-
- 1Cellomics Research Center, Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Korea. hglee@kribb.re.kr
- 2Department of Biology, Chungnam National University, Daejeon, Korea.
- 3Department of Laboratory Medicine, Konyang University, Nonsan, Korea.
- 4Department of Laboratory Medicine, Dankook University College of Medicine, Cheonan, Korea.
- KMID: 2238837
- DOI: http://doi.org/10.3343/kjlm.2006.26.4.287
Abstract
-
BACKGROUND: Human telomerase is a ribonucleoprotein polymerase, which synthesizes telomeric repeat sequences, and human telomerase reverse transcriptase (hTERT) has been identified as the catalytic subunit, as well as the rate-limiting component, of telomerase. In this study, we attempted to identify the modulators of telomerase, and to determine the molecular mechanisms underlying cisplatin-induced apoptosis.
METHODS
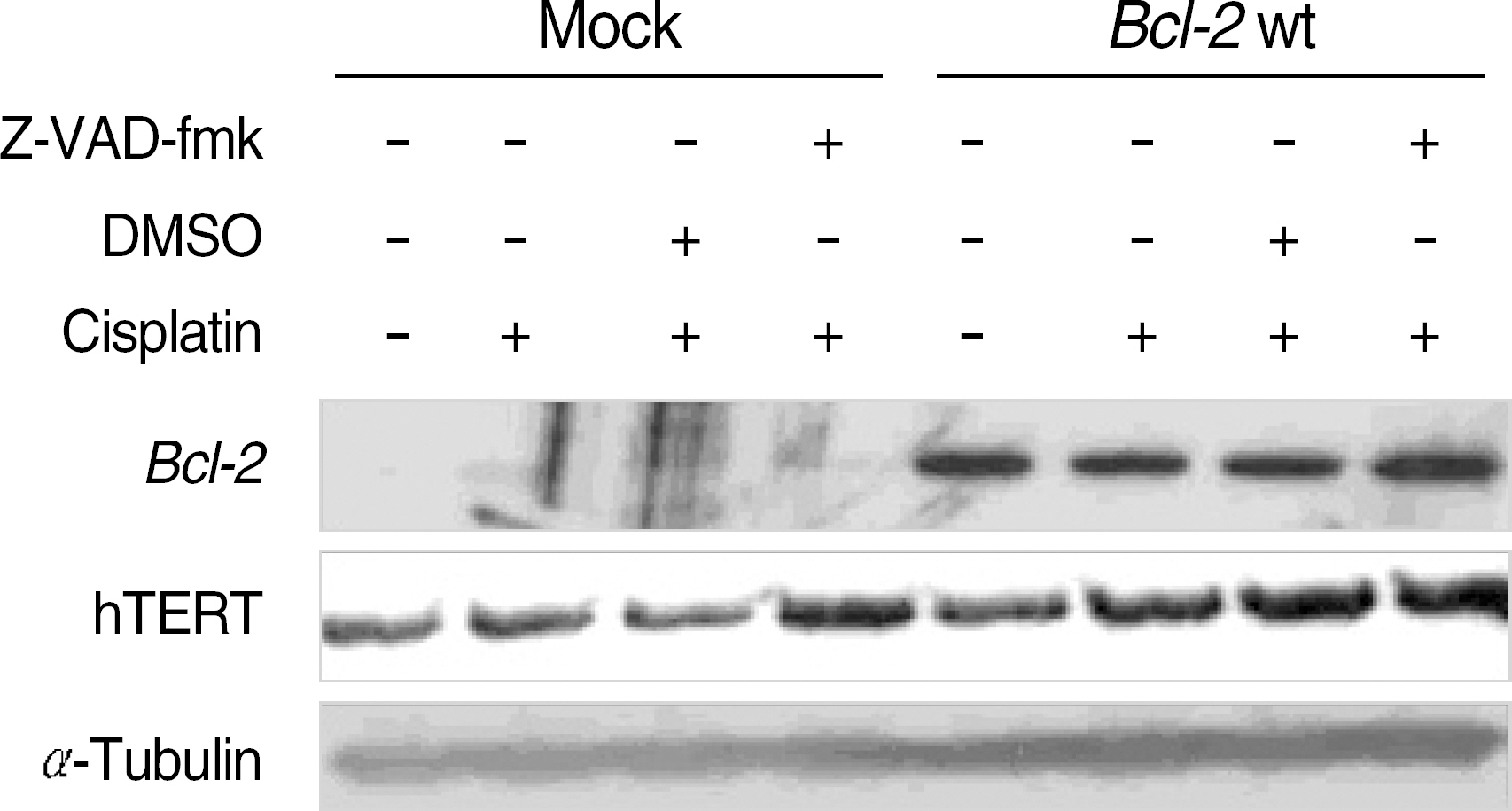
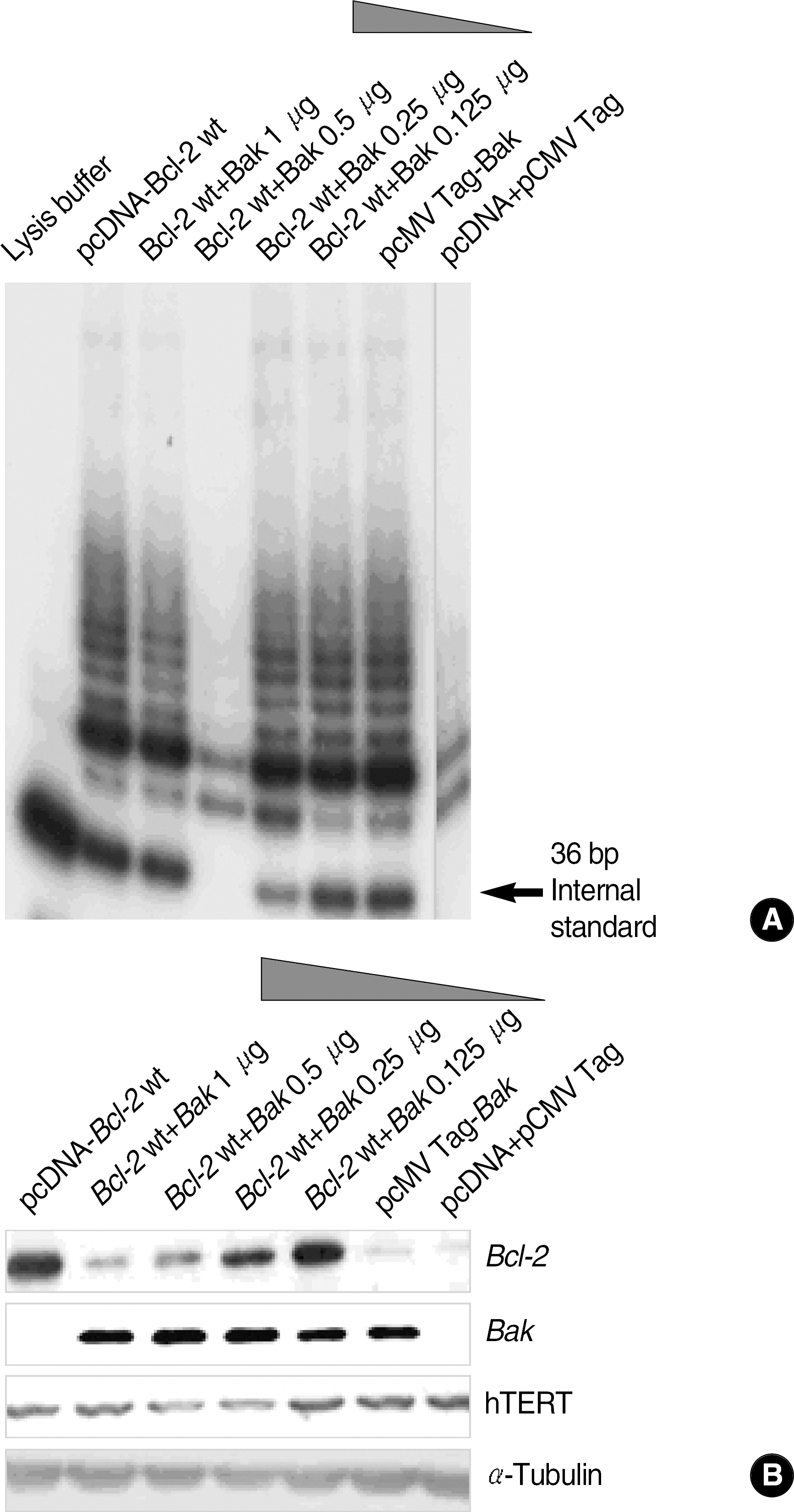
To determine the role of telomerase in cisplatin-induced apoptosis, we measured telomerase activity and analyzed apoptosis using PI and trypan blue staining. Also, we inhibited the caspase activations using Z-VAD-fmk to analyze the effects on expression of hTERT protein. Finally, we induced the transient co-expression of the Bcl-2 and Bak genes in HEK293 cells, and then, the telomerase activity and expression of hTERT were evaluated.
RESULTS
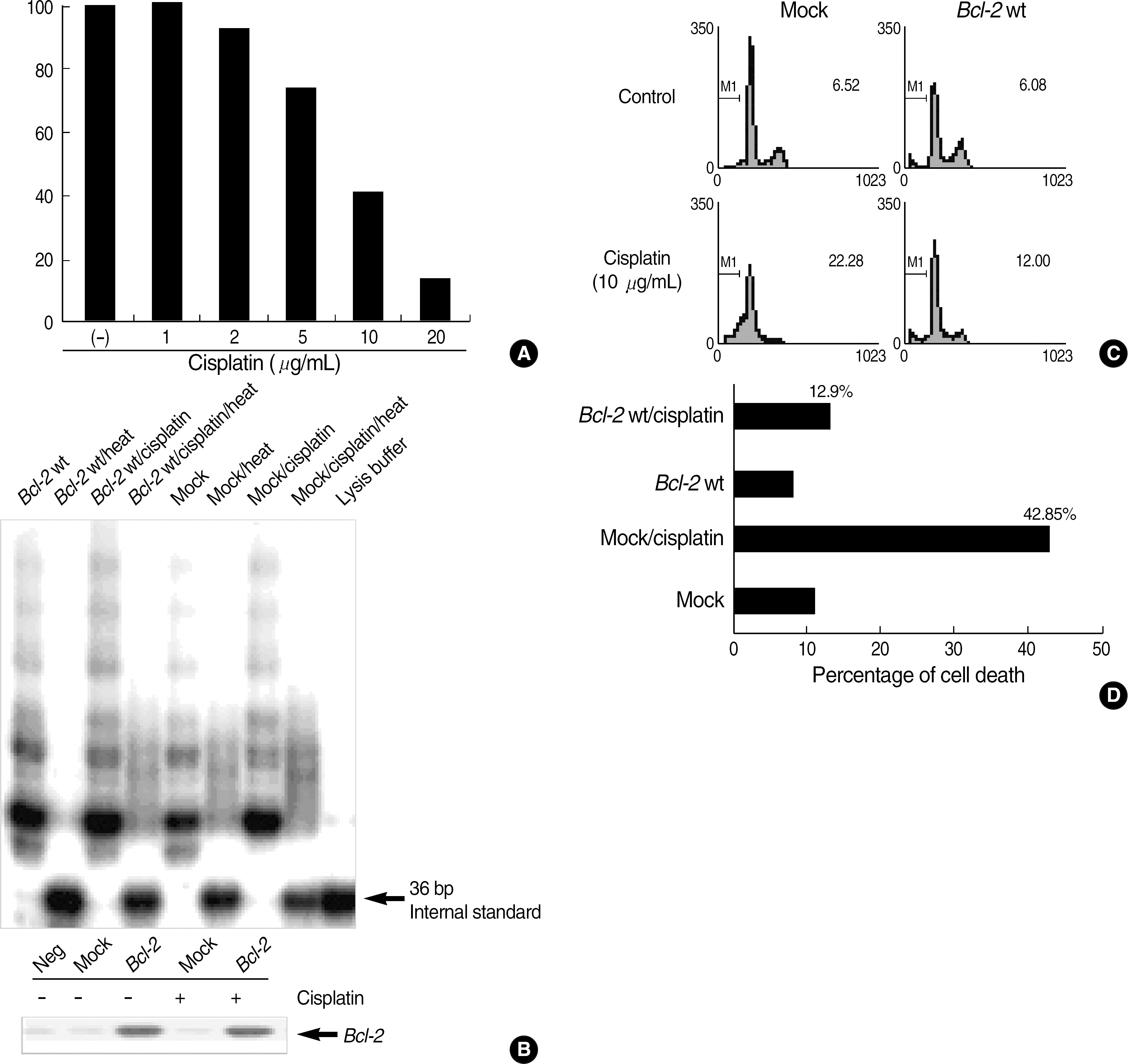
In the Bcl-2-overexpressing HeLa cells, telomerase activity was more enhanced, and cell death was reduced to 40-50% that of the mock controls. This finding suggests that Bcl-2-induced telomerase activity exerts an antiapoptotic effect in cisplatin-induced death. As caspase activation was inhibited via Z-VAD-fmk, the hTERT protein was recovered in the mock controls, but not in the Bcl-2-overexpressing cells. This suggests that the expression of hTERT can be regulated by caspases, but Bcl-2 was located within the upstream pathway. Moreover, when the Bcl-2 and Bak genes were co-transfected into the HEK293, both telomerase activity and hTERT protein were prominently reduced.
CONCLUSIONS
Bcl-2-induced telomerase activity inhibits cisplatin-induced apoptosis in HeLa cells, and can be regulated via both caspases and the interaction of Bcl-2 and Bak.
MeSH Terms
Figure
Reference
-
References
1. Blackburn EH. Structure and function of telomeres. Nature. 1991; 350:569–73.
Article2. Blackburn EH. Switching and signaling at the telomere.”. Cell. 2001; 106:661–73.
Article3. Stewart SA, Hahn WC, O'Connor BF, Banner EN, Lundberg AS, Modha P, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci USA. 2002; 99:12606–11.
Article4. Kondo Y, Kondo S, Tanaka Y, Haqqi T, Barna BP, Cowell JK. Inhibition of telomerase increases the susceptibility of human malignant glioblastoma cells to cisplatin-induced apoptosis. Oncogene. 1998; 16:2243–8.
Article5. Fu W, Begley JG, Killen MW, Mattson MP. Anti-apoptotic role of telomerase in pheochromocytoma cells. J Biol Chem. 1999; 274:7264–71.
Article6. Fu W, Killen M, Culmsee C, Dhar S, Pandita TK, Mattson MP. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J Mol Neurosci. 2000; 14:3–15.
Article7. Kushner DM, Paranjape JM, Bandyopadhyay B, Cramer H, Leaman DW, Kennedy AW, et al. 2–5A antisense directed against telomerase RNA produces apoptosis in ovarian cancer cells. Gynecol Oncol. 2000; 76:183–92.
Article8. Dudognon C, Pendino F, Hillion J, Saumet A, Lanotte M, Segal-Bendirdjian E. Death receptor signaling regulatory function for telomerase: hTERT abolishes TRAIL-induced apoptosis, independently of telomere maintenance. Oncogene. 2004; 23:7469–74.
Article9. Mattson MP, Klapper W. Emerging roles for telomerase in neuronal development and apoptosis. J Neurosci Res. 2001; 63:1–9.
Article10. Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell. 2001; 12:2023–30.
Article11. Ichiyoshi H, Kiyozuka Y, Kishimoto Y, Fukuhara S, Tsubura A. Massive telomere loss and telomerase RNA expression in dexamethasone-induced apoptosis in mouse thymocytes. Exp Mol Pathol. 2003; 75:178–86.
Article12. Zhang P, Chan SL, Fu W, Mendoza M, Mattson MP. TERT suppresses apoptotis at a premitochondrial step by a mechanism requiring reverse transcriptase activity and 14–3–3 protein-binding ability. FASEB J. 2003; 17:767–9.13. Gorbunova V, Seluanov A, Pereira-Smith OM. Expression of human telomerase (hTERT) does not prevent stress-induced senescence in normal human fibroblasts but protects the cells from stress-induced apoptosis and necrosis. J Biol Chem. 2002; 277:38540–9.
Article14. Holt SE, Glinsky VV, Ivanova AB, Glinsky GV. Resistance to apoptosis in human cells conferred by telomerase function and telomere stability. Mol Carcinogen. 1999; 25:241–8.
Article15. Mandal M, Kumar R. Bcl-2 modulates telomerase activity. J Biol Chem. 1997; 272:14183–7.16. MacNamara B, Wang W, Chen Z, Hou M, Mazur J, Gruber A, et al. Telomerase activity in relation to pro- and anti-apoptotic protein expression in high grade non-Hodgkin's lymphomas. Haematologica. 2001; 86:386–93.17. Loehrer PJ, Einhorn LH. Drugs five years later. Cisplatin. Ann Intern Med. 1984; 100:704–13.18. Chu G, Mantin R, Shen YM, Baskett G, Sussman H. Massive cisplatin overdose by accidental substitution for carboplatin. Toxicity and management. Cancer. 1993; 72:3707–14.
Article19. Folini M, Brambilla C, Villa R, Gandellini P, Vignati S, Paduano F, et al. Antisense oligonucleotide-mediated inhibition of hTERT, but not hTERC, induces rapid cell growth decline and apoptosis in the absence of telomere shortening in human prostate cancer cells. Eur J Cancer. 2005; 41:624–34.
Article20. Kraemer K, Fuessel S, Schmidt U, Kotzsch M, Schwenzer B, Wirth MP, et al. Antisense-mediated hTERT inhibition specifically reduces the growth of human bladder cancer cells. Clin Cancer Res. 2003; 9:3794–800.21. Tahara H, Shin-Ya K, Seimiya H, Yamada H, Tsuruo T, Ide T. G-Quadruplex stabilization by telomestatin induces TRF2 protein dissociation from telomeres and anaphase bridge formation accompanied by loss of the 3′ telomeric overhang in cancer cells. Oncogene. 2006; 25:1955–66.
Article22. Tauchi T, Shin-Ya K, Sashida G, Sumi M, Okabe S, Ohyashiki JH, et al. Telomerase inhibition with a novel G-quadruplex-interactive agent, telomestatin: in vitro and in vivo studies in acute leukemia. Oncogene. 2006. in press.
Article23. Pallini R, Sorrentino A, Pierconti F, Maggiano N, Faggi R, Montano N, et al. Telomerase inhibition by stable RNA interference impairs tumor growth and angiogenesis in glioblastoma xenografts. Int J Cancer. 2006; 118:2158–67.
Article24. Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994; 369:321–3.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Change of the Expression of Human Telomerase Reverse Transcriptase mRNA and Human Telomerase RNA after Cisplatin and 5-Fluorouracil Exposure in Head and Neck Squamous Cell Carcinoma Cell Lines
- Telomerase Activity and Expression of hTR and TERT in Human Soft Tissue Sarcomas
- Effect of Bcl-2 Expression and Telomerase Activity on Apoptosis of the Brain Tumors
- The Change of Telomerase Activity by Cisplatin and 5-FU in Head and Neck Cancer Cell Lines
- The Expression of Telomerase Reverse Transcriptase Protein is an Independent Prognostic Marker in Early Stage Non-Small Cell Lung Carcinomas