Korean Diabetes J.
2008 Apr;32(2):131-140. 10.4093/kdj.2008.32.2.131.
Cloning of Novel Epidermal Growth Factor (EGF) Plasmid for Gene Therapy on Diabetic Foot Ulcer
- Affiliations
-
- 1Molecular Therapy Lab, Paik Memorial Institute for Clinical Research, Korea.
- 2Department of Internal Medicine, Inje College of Medicine, Korea.
- 3Department of Internal Medicine, Maryknoll General Hospital, Korea.
- KMID: 2222509
- DOI: http://doi.org/10.4093/kdj.2008.32.2.131
Abstract
-
BACKGROUND: Epidermal Growth Factor (EGF) is one of the important growth factors involved in the epithelialization during cutaneous wound healing. Peptide EGF has been used for the treatment of diabetic foot ulcer. But the inferiority of cost-effectiveness and the inconvenience of daily application might have restricted its wide clinical usage. EGF gene therapy could dramatically improve the efficacy and inconvenience through long-term expression and bypassing the EGF degradation by hostile non-specific proteinases expressed in the wound bed.
METHODS
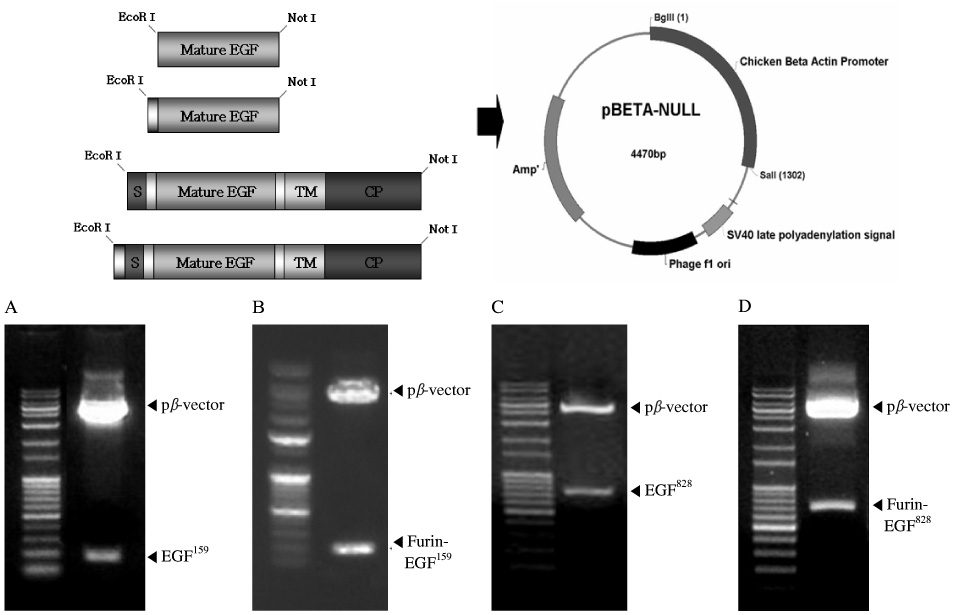
EGF DNAs were amplified via PCR. For the more effective secretion from the transfected cell, we inserted furin cleavage site into EGF plasmids. The efficacy of novel plasmid pbeta-EGF was verified by transfection into the various animal cell lines, and the biologic potency of expressed EGF was confirmed via phosphorylation of PI3K and GSK3beta by Western blotting.
RESULTS
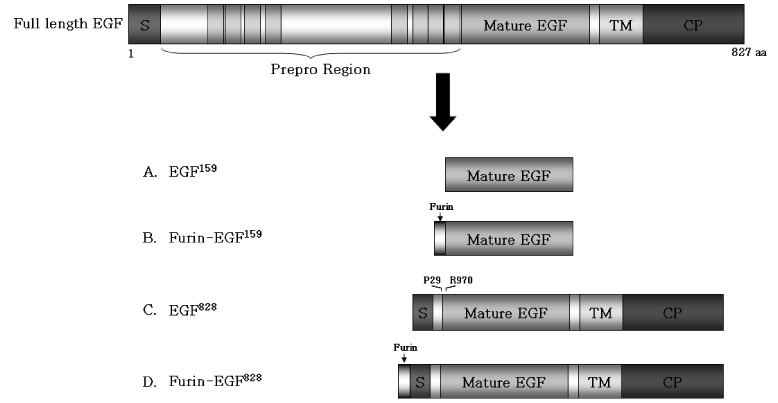
We tested various kinds of human EGFs. One of the human EGF isoforms, EGF(828) including a membrane-anchoring domain was successfully released as the mature EGF protein in the cell culture media. Also EGF plasmid including furin cleavage site showed more than 2-fold increased EGF expression compared with the sequence without furin cleavage site.
CONCLUSION
In conclusion, these findings suggest that mature EGF could be released easily out of cells by modifying EGF DNA sequence. Our novel EGF plasmid DNA could markedly increase the efficiency of non-viral gene therapy for diabetic foot ulcer.
MeSH Terms
-
Animals
Base Sequence
Cell Culture Techniques
Cell Line
Clone Cells
Cloning, Organism
Diabetes Mellitus
Diabetic Foot
DNA
Epidermal Growth Factor
Furin
Genetic Therapy
Glycogen Synthase Kinase 3
Humans
Intercellular Signaling Peptides and Proteins
Peptide Hydrolases
Phosphorylation
Plasmids
Polymerase Chain Reaction
Protein Isoforms
Transfection
Ulcer
Wound Healing
DNA
Epidermal Growth Factor
Furin
Glycogen Synthase Kinase 3
Intercellular Signaling Peptides and Proteins
Peptide Hydrolases
Protein Isoforms
Figure
Reference
-
1. Grotendorst GR, Martin GR, Pencev D, Sodek J, Harvey AK. Stimulation of granulation tiussue formation by platelet-derived growth factor in normal and diabetic rats. J Clin Invest. 1985. 76:2323–2329.2. Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RN, Deuel TF. Platelet-derived growth factor and transforming growth factor-β enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989. 109:429–440.3. Knighton DR, Phillips GD, Fiegel VD. Wound healing angiogenesis: indirect stimulation by basic fibroblast growth factor. J Trauma. 1990. 30:S134–S144.4. Staiano-Coico L, Krueger JG, Rubin JS, D'limi S, Vallat VP, Valentino L, Fahev T 3rd, Hawes A, Kingston G, Madden MR. Human keratinocyte growth factor effects in a porcine model of epidermal wound healing. J Exp Med. 1993. 178:865–878.
Article5. Werner S, Breeden M, Hubner G, Greenhalqh DG, Longaker MT. Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. J Invest Dermatol. 1994. 103:469–473.
Article6. Sotozono C, Inatomi T, Nakamura M, Kinoshita S. Keratinocyte growth factor accelerates corneal epithelial wound healing in vivo. Invest Ophthalmal Vis Sci. 1995. 36:1524–1529.7. Kopp J, Wang GY, Kulmburg P, Schultze-Mosgau S, Huan JN, Ying K, Seyhan H, Jeschke MD, Kneser U, Bach AD, Ge SD, Doolev S, Horch RE. Accelerated wound healing by in vivo application of keratinocytes overexpressing KGF. Mol Ther. 2004. 10:86–96.
Article8. Broadley KN, Aquino AM, Hicks B, Ditesheim JA, McGee GS, Demetriou AA, Woodward SC, Davidson JM. Growth factors bFGF and TGF beta accelerate the rate of wound repair in normal and in diabetic rats. Int J Tissue React. 1988. 10:345–353.9. Quaglino D Jr, Nanney LB, Ditesheim JA, Davidson JM. Transforming growth factor-beta stimulates wound healing and modulates extracellular matrix gene expression in pig skin: incisional wound model. J Invest Dermatol. 1991. 97:34–42.10. Greenhalgh DG. The role of growth factors in wound healing. J Trauma. 1996. 41:159–167.
Article11. Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003. 90:133–146.
Article12. Deodato B, Arsic N, Zentilin L, Galeano M, Santoro D, Torre V, Altavilla D, Valdembri D, Bussolino F, Squadrito F, Giacca M. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene. 2002. 9:777–785.
Article13. Romano Di Peppe S, Mangoni A, Zambruno G, Spinetti G, Melillo G, Napolitano M, Capogrossi MC. Adenovirus-mediated VEGF(165) gene transfer enhances wound heaing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 2002. 9:1271–1277.14. Taylor JM, Mitchell WM, Cohen S. Epidermal growth factor. Physical and chemical properties. J Biol Chem. 1972. 247:5928–5934.15. Savage CR Jr, Hash JH, Cohen S. Epidermal growth factor: Location of disulfide bonds. J Biol Chem. 1973. 248:7669–7672.16. Gregory H. Isolation and structure of urogastrone and its relationship to epidermal growth factor. Nature. 1975. 257:325–327.
Article17. Cohen S, Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci USA. 1975. 72:1317–1321.
Article18. Servold SA. Growth factor impact on wound healing. Clin Podiatr Med Surg. 1991. 8:937–953.19. Brown GL, Curtsinger L 3rd, Brightwell JR, Ackerman DM, Tobin GR, Polk HC Jr, George-Nascimento C, Valenzuela P, Schultz GS. Enhancement of epidermal regeneration by biosynthetic epidermal growth factor. J Exp Med. 1986. 163:1319–1324.
Article20. Carpenter G, Cohens S. Epidermal growth factor. Annu Rev Biochem. 1979. 48:193–216.
Article21. Andree C, Swain WF, Page CP, Macklin MD, Slama J, Hatzis D, Eriksson E. In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci USA. 1994. 91:12188–12192.
Article22. Brazzell RK, Stern ME, Aquavella JV, Beuerman RW, Baird L. Human recombinant epidermal growth factor in experimental corneal wound healing. Invest Ophthalmol Vis Sci. 1991. 32:336–340.23. Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg. 2006. 56:394–398.
Article24. Thorne BA, Plowman GD. The heparin-binding domain of amphiregulin necessitates the precursor pro-region for growth factor secretion. Mol Cell Bio. 1994. 14:1635–1646.
Article25. Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, Mekada E, Taniguchi N. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995. 6:967–980.
Article26. Saito Y, Vandenheede JR, Cohen P. The mechanism by which epidermal growth factor inhibits glycogen synthase kinase 3 in A431 cells. Biochem J. 1994. 303:27–31.
Article27. Konturek A, Barczynski M, Cichon S, Pituch-Noworolska A, Jonkisz J, Cinhon W. Significance of vascular endothelial growth factor and epidermal growth factor in development of papillary thyroid cancer. Langenbecks Arch Surg. 2005. 390:216–221.
Article28. Xu K, Shu HK. EGFR activation results in enhanced cyclooxygenase-2 expression through p38 mitogen-activated protein kinase-dependent activation of the Sp1/Sp3 transcription factors in human gliomas. Cancer Res. 2007. 67:6121–6129.
Article29. Rothhut B, Ghoneim C, Antonicelli F, Soula-Rothhut M. Epidermal growth factor stimulates matrix metalloproteinase-9 expression and invasion in human follicular thyroid carcinoma cells through Focal adhesion kinase. Biochimie. 2007. 89:613–624.
Article30. Garrett TP, McKern NM, Lou M, Elleman TC, Adams TE, Lovrecz GO, Zhu HJ, Walker F, Frenkel MJ, Hoyne PA, Jorissen RN, Nice EC, Burgess AW, Ward CW. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002. 110:763–773.31. Britsch S, Li L, Kirchhoff S, Theuring J, Brinkmann V, Birchmeier C, Riethmacher D. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev. 1998. 12:1825–1836.32. Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007. 190:1–65.33. Toyoda H, Komurasaki T, Uchida D, Takavama Y, Isobe T, Okuyama T, Hanada K. Epiregulin. A novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J Biol Chem. 1995. 270:7495–7500.34. Wong ST, Winchell LF, McCune BK, Earp HS, Teixido J, Massague J, Herman B, Lee DC. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989. 56:495–506.35. Wiley HS, Woolf MF, Opresko LK, Burke PM, Will B, Morgan JR, Lauffenburger DA. Removal of the membrane-anchoring domain of epidermal growth factor leads to intracrine signaling and disruption of mammary epithelial cell organization. J Cell Biol. 1998. 143:1317–1328.
Article36. Le Gall SM, Auger R, Dreux C, Mauduit P. Regulated cell surface pro-EGF ectodomain shedding is a zinc metalloprotease-dependent process. J Biol Chem. 2003. 278:45255–45268.
Article37. Wong RW, Kwan RW, Mak PH, Mak KK, Sham MH, Chan SY. Overexpression of epidermal growth factor induced hypospermatogenesis in transgenic mice. J Biol Chem. 2000. 275:18297–18301.
Article38. Dong J, Wiley HS. Trafficking and proteolytic release of epidermal growth factor receptor ligands are modulated by their membrane-anchoring domains. J Biol Chem. 2000. 275:557–564.
Article39. Creemers JW, Vey M, Schafer W, Ayoubi TA, Roebroek AJ, Klenk HD, Garten W, Van de Ven WJ. Endoproteolytic cleavage of its propeptide is a prerequisite for efficient transport of furin out of the endoplasmic reticulum. J Biol Chem. 1995. 270:2695–2702.
Article40. Hosaka M, Nagahama M, Kim WS, Watanabe T, Hatsuzawa K, Ikemizu J, Murakami K, Nakyama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991. 266:12127–12130.
Article41. Deodato B, Arsic N, Zentilin L, Galeano M, Santoro D, Torre V, Altavilla D, Valdembri D, Bussolino F, Squadrito F, Giacca M. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther. 2002. 9:777–785.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Epidermal Growth Factor at Ulcer Margin and Antral Mucosa in Patients with Gastric Ulcer
- Comparison of Minicircle with Conventional Plasmid for the Non-viral Vascular Endothelial Growth Factor (VEGF) Gene Therapy
- Expression of Epidermal Growth Factor, Transforming Growth Factor-alphaand Epidermal Growth Factor Receptor in Human Trophoblast and Decidua
- Study on the Effects of Epidermal Growth Factor on Epidermal and Hair Growth in the Mouse
- An experimental study on the stimulatory effects of epidermal growth factor and transforming growth factor-alpha on the growth of squamous cancer cell lines