J Korean Diabetes Assoc.
2007 Nov;31(6):465-471. 10.4093/jkda.2007.31.6.465.
Comparison of Minicircle with Conventional Plasmid for the Non-viral Vascular Endothelial Growth Factor (VEGF) Gene Therapy
- Affiliations
-
- 1Paik Diabetes Center, Division of Endocrinology & Metabolism, Pusan Paik Hospital, College of Medicine, Inje University.
- 2Molecular Therapy Lab, Paik Memorial Institute for Clinical Research, Inje University.
- 3Department of Internal Medicine, Maryknoll General Hospital.
- KMID: 2177559
- DOI: http://doi.org/10.4093/jkda.2007.31.6.465
Abstract
-
BACKGROUND: Delayed wound healings in diabetic patients are related with the impairment of the expressions of various growth factors. Treatments using growth factors have been attempted on diabetic foot ulcer. VEGF (vascular endothelial growth factor) accelerates neo-angiogenesis on the early phase of the wound healing and exerts chemo-attractive effect for the other growth factors and cytokines. Non-viral gene transfer strategies are attractive tool for the gene therapy due to the safety and the versatility, but the low efficiency has been the serious problem.
METHODS
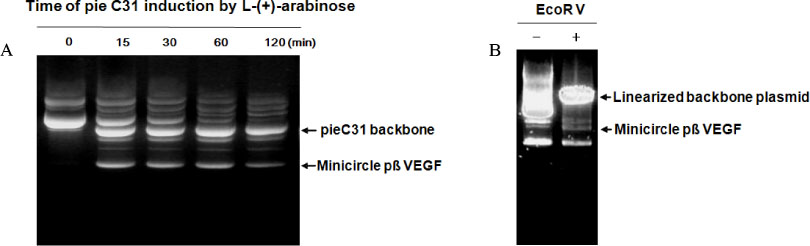
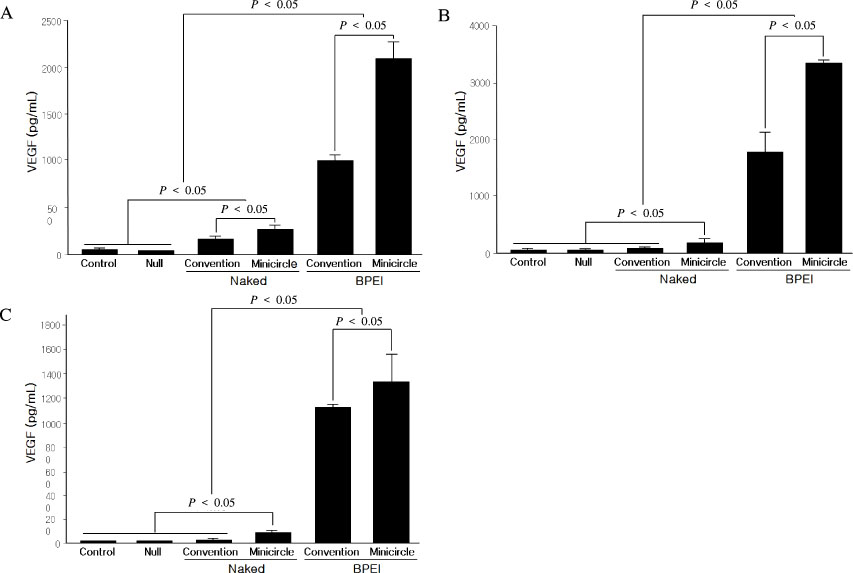
We performed the VEGF gene therapy using reconstructed minicircle MINI-pbetaVEGF DNA with a polymeric carrier, polyethylenimine (PEI, 25 kDa) in HEK293, CHO, and NIH3T3 cell lines, and compared its efficiency with the conventional VEGF plasmid pbetaVEGF.
RESULTS
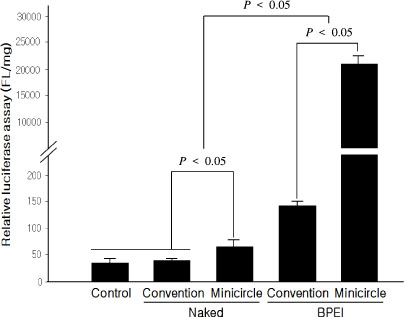
The levels of expressed VEGF were higher in the groups using BPEI (branched PEI) as a gene carrier than naked plasmid transfer in all cell lines (P < 0.05). The minicircle MINI-pbetaVEGF DNA showed much higher VEGF expression than conventional plasmid pbetaVEGF (P < 0.05).
CONCLUSION
Minicircle DNA MINI-pbetaVEGF showed much higher transfection efficiency than conventional plasmid pbetaVEGF. It might be used in actual human clinical trial due to its higher efficiency and possible safety for the treatment of diabetic foot ulcer.
MeSH Terms
-
Cell Line
Cytokines
Diabetic Foot
DNA
Genes, vif
Genetic Therapy*
Humans
Intercellular Signaling Peptides and Proteins
Plasmids*
Polyethyleneimine
Polymers
Transfection
Ulcer
Vascular Endothelial Growth Factor A*
Wound Healing
Cytokines
DNA
Intercellular Signaling Peptides and Proteins
Polyethyleneimine
Polymers
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004. 27:1047–1053.2. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005. 366:1719–1724.4. Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005. 366:1736–1743.5. Bennett SP, Griffiths GD, Schor AM, Leese GP, Schor SL. Growth factors in the treatment of diabetic foot ulcers. Br J Surg. 2003. 90:133–146.6. Harding KG, Morris HL, Patel GK. Science, medicine and the future: healing chronic wounds. BMJ. 2002. 324:160–163.7. Pollard H, Remy JS, Loussouam G, Demolombe S, Behr JP, Escande D. Polyethylenimine but not cationic lipids promotes transgene delivery to the nucleus in mammalian cells. J Biol Chem. 1998. 273:7507–7511.8. Sonawane ND, Szoka FC, Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003. 278:44826–44831.9. Darquet AM, Rangara R, Kreiss P, Schwartz B, Naimi S, Delaere P, Crouzet J, Scherman D. Minicircle: an improved DNA molecule for in vitro and in vivo gene transfer. Gene Ther. 1999. 6:209–218.10. Bigger BW, Tolmachov O, Collombet JM, Fragkos M, Palaszewski I, Coutelle C. An ARAC-controlled bacterial CRE expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J Biol Chem. 2001. 276:23018–23027.11. Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003. 8:495–500.12. Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007. 117:1219–1222.13. Iruela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost. 1997. 78:672–677.14. Kirchner LM, Meerbaum SO, Gruber BS, Knoll AK, Bulgrin J, Taylor RAJ, Schmidt SP. Effects of vascular endothelial growth factor on wound closure rates in the genetically diabetic mouse model. Wound Repair Regen. 2003. 11:127–131.15. Pandya NM, Dhalla NS, Santani DD. Angiogenesis-a new target for future therapy. Vascul Pharmacol. 2006. 44:265–274.16. Frank S, Hbuner G, Breier G, Longaker M, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. J Biol Chem. 1995. 270:12607–12613.17. Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995. 92:7297–7301.18. Thomas M, Klibanov AM. Enhancing polyethylenimine's delivery of plasmid DNA into mammalian cells. Proc Natl Acad Sci USA. 2002. 99:14640–14645.19. Furgeson DY, Chan WS, Yockman JW, Kim SW. Modified linear polyethylenimine-cholesterol conjugates for DNA complexation. Bioconjug Chem. 2003. 14:840–847.20. Nettelbeck DM, Jerome V, Muller R. A strategy for enhancing the transcriptional activity of weak cell type-specific promoters. Gene Ther. 1998. 5:1656–1664.21. Natesan S, Molinari E, Rivera VM, Rickles RJ, Gilman M. A general strategy to enhance the potency of chimeric transcriptional activators. Proc Natl Acad Sci USA. 1999. 96:13898–13903.22. Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 1997. 4:1341–1349.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of EGF with VEGF Non-Viral Gene Therapy for Cutaneous Wound Healing of Streptozotocin Diabetic Mice
- Improved expression by cytomegalovirus promoter/enhancer and behavior of vascular endothelial growth factor gene after myocardial injection of naked DNA
- Expression of Vascular Endothelial Growth Factor in Hepatocellular Carcinomas
- Expression of Placenta Growth Factor in the Oral Squamous Cell Carcinoma
- Expression of Vascular Endothelial Growth Factors A,C and D in Gastric Adenocarcinoma