Korean Diabetes J.
2010 Jun;34(3):157-165. 10.4093/kdj.2010.34.3.157.
The Changes in Early Phase Insulin Secretion in Newly Diagnosed, Drug Naive Korean Prediabetes Subjects
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Kyung Hee University School of Medicine, Seoul, Korea. jtwoomd@khmc.or.kr
- 2Research Institute of Endocrinology, Kyung Hee University, Seoul, Korea.
- 3Department of Internal Medicine, Dongsuwon Hospital, Suwon, Korea.
- KMID: 2222371
- DOI: http://doi.org/10.4093/kdj.2010.34.3.157
Abstract
- BACKGROUND
There have been no systematic observations regarding changes in early phase insulin secretion among Korean prediabetes and early stage type 2 diabetes mellitus (T2DM) patients.
METHODS
We conducted 75-g oral glucose tolerance tests (OGTT) in 873 subjects with suspected abnormal glucose tolerance. All subjects were diagnosed as having normal glucose tolerance (NGT), prediabetes (preDM), or T2DM according to the OGTT results and the insulin secretory and insulin resistance indices of each subject were calculated. Additionally, we analyzed the changes in early phase insulin secretion according to changes in fasting (Glc(0)), post-prandial (Glc(120)) glucose and HbA1c (A1c) levels.
RESULTS
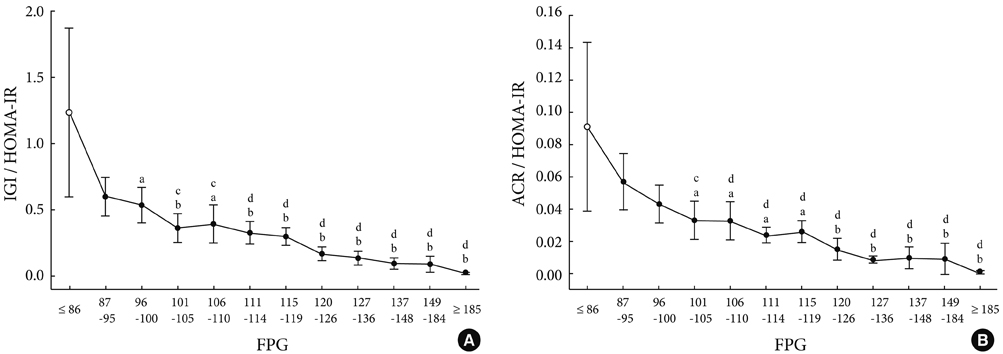
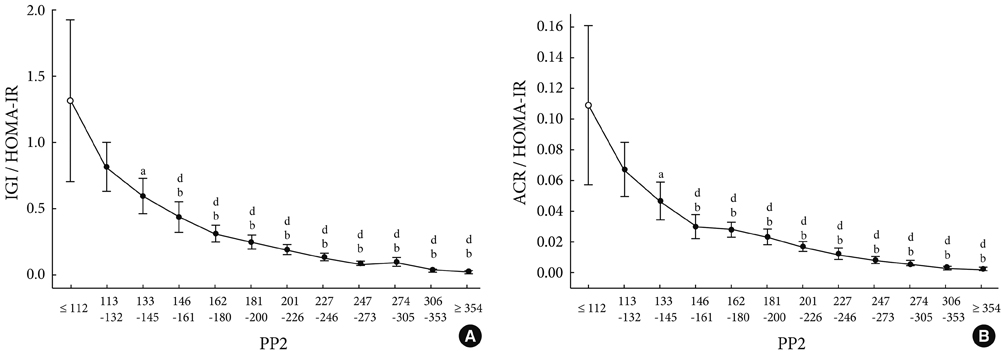
As compared to subjects with NGT, the insulin secretory indices of the preDM and T2DM subjects progressively declined, and the insulin resistance indices were progressively aggravated. Early phase insulin secretion decreased rapidly according to the increments of Glc(0), Glc(120) and A1c, and these changes were most prominent in the NGT stage. Compared to the control group, the early phase insulin secretion levels of the preDM or T2DM subjects were less than 50% when Glc(0) was over 100 mg/dL, Glc(120) was over 145 mg/dL, and A1c was over 5.8%.
CONCLUSION
This study suggests that progressive beta cell dysfunction in Koreans may be initiated and rapidly aggravated during the period generally designated as 'normal.'
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Hospital-Based Korean Diabetes Prevention Study: A Prospective, Multi-Center, Randomized, Open-Label Controlled Study
Sang Youl Rhee, Suk Chon, Kyu Jeung Ahn, Jeong-Taek Woo,
Diabetes Metab J. 2019;43(1):49-58. doi: 10.4093/dmj.2018.0033.
Reference
-
1. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004. 88:787–835.2. UKPDS study group. U.K. Prospective diabetes study 16. Overview of 6 years' therapy of type ii diabetes: a progressive disease. U.K. Prospective diabetes study group. Diabetes. 1995. 44:1249–1258.3. Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999. 104:787–794.4. Lillioja S, Mott DM, Howard BV, Bennett PH, Yki-Jarvinen H, Freymond D, Nyomba BL, Zurlo F, Swinburn B, Bogardus C. Impaired glucose tolerance as a disorder of insulin action: longitudinal and cross-sectional studies in pima Indians. N Engl J Med. 1988. 318:1217–1225.5. Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH. Sequential changes in serum insulin concentration during development of non-insulin-dependent diabetes. Lancet. 1989. 1:1356–1359.6. Hansen BC, Bodkin NL. Heterogeneity of insulin responses: phases leading to type 2 (non-insulin-dependent) diabetes mellitus in the rhesus monkey. Diabetologia. 1986. 29:713–719.7. Reaven GM, Hollenbeck CB, Chen YD. Relationship between glucose tolerance, insulin secretion, and insulin action in non-obese individuals with varying degrees of glucose tolerance. Diabetologia. 1989. 32:52–55.8. Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001. 86:4047–4058.9. Bergman RN, Finegood DT, Kahn SE. The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes. Eur J Clin Invest. 2002. 32:Suppl 3. 35–45.10. American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997. 20:1183–1197.11. Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, Knowler WC. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000. 23:1113–1118.12. DECODE study group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001. 161:397–405.13. Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care. 2001. 24:1397–1402.14. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004. 27:Suppl 1. S5–S10.15. Forouhi NG, Balkau B, Borch-Johnsen K, Dekker J, Glumer C, Qiao Q, Spijkerman A, Stolk R, Tabac A, Wareham NJ. The threshold for diagnosing impaired fasting glucose: a position statement by the European Diabetes Epidemiology Group. Diabetologia. 2006. 49:822–827.16. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. 2004. 47:31–39.17. Godsland IF, Jeffs JA, Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia. 2004. 47:1157–1166.18. Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005. 353:1454–1462.19. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab. 2003. 88:2300–2308.20. Rhee SY, Chon S, Oh S, Kim SW, Kim JW, Kim YS, Woo JT. Insulin secretion and insulin resistance in newly diagnosed, drug naive prediabetes and type 2 diabetes patients with/without metabolic syndrome. Diabetes Res Clin Pract. 2007. 76:397–403.21. Rhee SY, Kwon MK, Park BJ, Chon S, Jeong IK, Oh S, Ahn KJ, Chung HY, Kim SW, Kim JW, Kim YS, Woo JT. Differences in insulin sensitivity and secretory capacity based on OGTT in subjects with impaired glucose regulation. Korean J Intern Med. 2007. 22:270–274.22. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001. 50:590–593.23. Kosaka K, Kuzuya T, Hagura R, Yoshinaga H. Insulin response to oral glucose load is consistently decreased in established non-insulin-dependent diabetes mellitus: the usefulness of decreased early insulin response as a predictor of non-insulin-dependent diabetes mellitus. Diabet Med. 1996. 13:9 Suppl 6. S109–S119.24. Kosaka K, Hagura R, Kuzuya T, Kuzuya N. Insulin secretory response of diabetics during the period of improvement of glucose tolerance to normal range. Diabetologia. 1974. 10:775–782.25. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.26. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000. 85:2402–2410.27. Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002. 51:2170–2178.28. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009. 32:1327–1334.29. Muscelli E, Mari A, Natali A, Astiarraga BD, Camastra S, Frascerra S, Holst JJ, Ferrannini E. Impact of incretin hormones on beta-cell function in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2006. 291:E1144–E1150.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Insulin Secretion and Insulin Resistance in Newly Diagnosed, Drug Naive Prediabetes and Type 2 Diabetes Patients With/Without Metabolic Syndrome
- Retraction: Insulin Secretion and Insulin Resistance in Newly Diagnosed, Drug Naive Prediabetes and Type 2 Diabetes Patients With/Without Metabolic Syndrome
- Changing Clinical Characteristics according to Insulin Resistance and Insulin Secretion in Newly Diagnosed Type 2 Diabetic Patients in Korea
- Impact of Serum Triglyceride and High Density Lipoprotein Cholesterol Levels on Early-Phase Insulin Secretion in Normoglycemic and Prediabetic Subjects
- Association of Protein Z with Prediabetes and Type 2 Diabetes