Ann Rehabil Med.
2013 Jun;37(3):301-310. 10.5535/arm.2013.37.3.301.
The Effect of Continuous Epidural Electrical Stimulation on Neuronal Proliferation in Cerebral Ischemic Rats
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation & Regional Cerebro-Cardiovascular Center, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea. cyang@ricres.org
- 2Department of Physical Medicine and Rehabilitation, Design Hospital, Jeonju, Korea.
- 3Rehabilitation Institute of Chicago and Northwestern University, Chicago, IL, USA.
- 4Department of Neurosurgery, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea.
- 5Department of Pharmacology, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea.
- 6Department of Nuclear Medicine, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea.
- 7Department of Anatomy, Chonbuk National University Medical School, Jeonju, Korea.
- KMID: 2219526
- DOI: http://doi.org/10.5535/arm.2013.37.3.301
Abstract
OBJECTIVE
To investigate the effect of electrical stimulation (ES) on the recovery of motor skill and neuronal cell proliferation.
METHODS
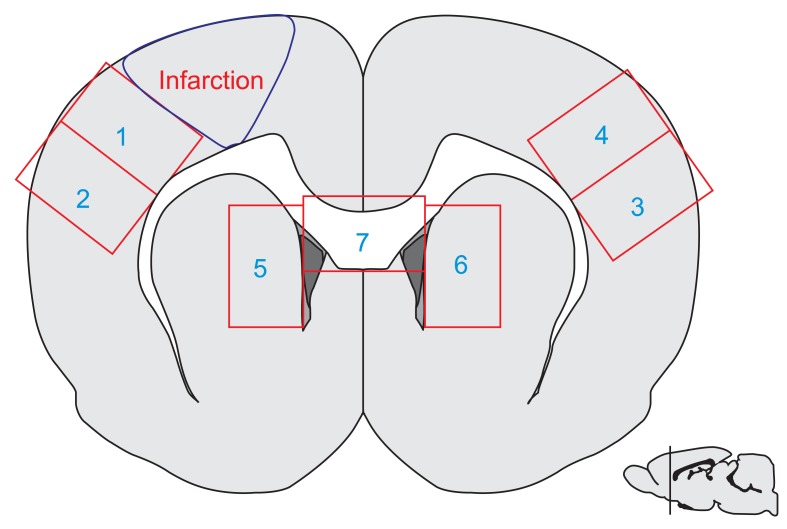
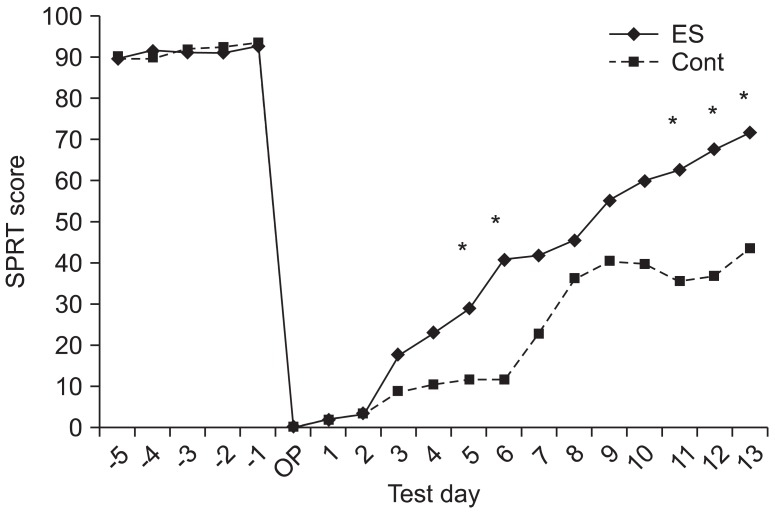
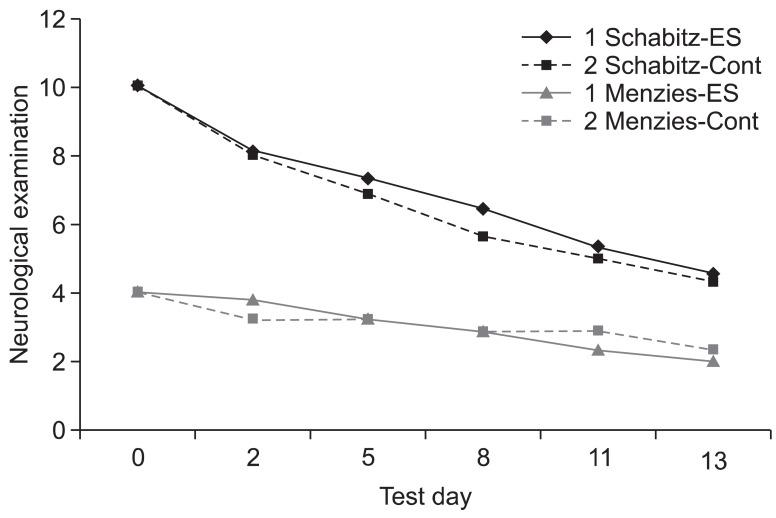
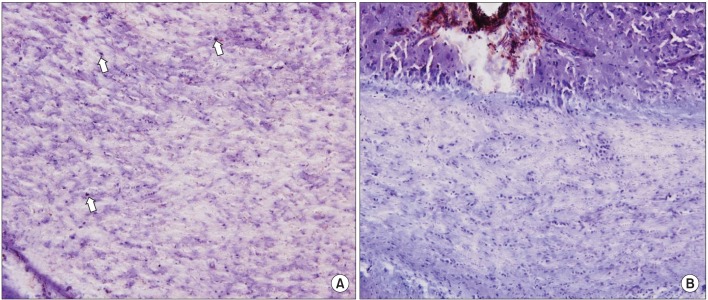
The male Sprague-Dawley rats were implanted with an epidural electrode over the peri-ischemic area after photothrombotic stroke in the dominant sensorimotor cortex. All rats were randomly assigned into the ES group and control group. The behavioral test of a single pellet reaching task (SPRT) and neurological examinations including the Schabitz's photothrombotic neurological score and the Menzies test were conducted for 2 weeks. After 14 days, coronal sections were obtained and immunostained for neuronal cell differentiation markers including bromodeoxyuridine (BrdU), neuron-specific nuclear protein (NeuN), and doublecortin (DCX).
RESULTS
On the SPRT, the motor function in paralytic forelimbs of the ES group was significantly improved. There were no significant differences in neurological examinations and neuronal cell differentiation markers except for the significantly increased number of DCX+ cells in the corpus callosum of the ES group (p<0.05). But in the ES group, the number of NeuN+ cells in the ischemic cortex and the number of NeuN+ cells and DCX+ cells in the ischemic striatum tended to increase. In the ES group, NeuN+ cells in the ischemic hemisphere and DCX+ cells and BrdU+ cells in the opposite hemisphere tended to increase compared to those in the contralateral.
CONCLUSION
The continuous epidural ES of the ischemic sensorimotor cortex induced a significant improvement in the motor function and tended to increase neural cell proliferation in the ischemic hemisphere and the neural regeneration in the opposite hemisphere.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Electric Cortical Stimulation (ECS) and Transcranial Direct Current Stimulation (tDCS) on Rats With a Traumatic Brain Injury
Ki Pi Yu, Yong-Soon Yoon, Jin Gyeong Lee, Ji Sun Oh, Jeong-Seog Lee, Taeyong Seog, Han-Young Lee
Ann Rehabil Med. 2018;42(4):502-513. doi: 10.5535/arm.2018.42.4.502.
Reference
-
1. Statistics Korea. Annual reports on the cause of death statistics 2010. Deajeon: Statistics Korea;2011.2. Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien). 1991; 52:137–139. PMID: 1792954.
Article3. Brown JA, Lutsep H, Cramer SC, Weinand M. Motor cortex stimulation for enhancement of recovery after stroke: case report. Neurol Res. 2003; 25:815–818. PMID: 14669524.
Article4. Brown JA, Lutsep HL, Weinand M, Cramer SC. Motor cortex stimulation for the enhancement of recovery from stroke: a prospective, multicenter safety study. Neurosurgery. 2006; 58:464–473. PMID: 16528186.
Article5. Franzini A, Ferroli P, Dones I, Marras C, Broggi G. Chronic motor cortex stimulation for movement disorders: a promising perspective. Neurol Res. 2003; 25:123–126. PMID: 12635509.
Article6. Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002; 22:6631–6638. PMID: 12151542.
Article7. Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003; 9:261–272. PMID: 12934709.
Article8. Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, McGeer PL, et al. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000; 41:10–18. PMID: 10643917.
Article9. Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol Res. 2003; 25:780–788. PMID: 14669519.
Article10. Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004; 24:628–633. PMID: 14736848.
Article11. Plautz EJ, Barbay S, Frost SB, Friel KM, Dancause N, Zoubina EV, et al. Post-infarct cortical plasticity and behavioral recovery using concurrent cortical stimulation and rehabilitative training: a feasibility study in primates. Neurol Res. 2003; 25:801–810. PMID: 14669522.
Article12. Yang CY, Moon SK, Song JH, Kim HS, Han EH, Kim TJ, et al. The effect of continuous epidural electrical stimulation on synapse and neuronal cell in rat with focal ischemia. J Korean Acad Rehabil Med. 2008; 32:375–387.13. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Waltham: Academic Press;2004.14. Watson BD, Dietrich WD, Busto R, Wachtel MS, Ginsberg MD. Induction of reproducible brain infarction by photochemically initiated thrombosis. Ann Neurol. 1985; 17:497–504. PMID: 4004172.
Article15. Moon SK, Yang CY, No SE, Kim EY, Lee S, Park SA, et al. Promotion of motor recovery by anodal continuous and low amplitude cortical stimulation in rat stroke model. Lab Anim Res. 2007; 23:25–30.16. Adkins DL, Campos P, Quach D, Borromeo M, Schallert K, Jones TA. Epidural cortical stimulation enhances motor function after sensorimotor cortical infarcts in rats. Exp Neurol. 2006; 200:356–370. PMID: 16678818.
Article17. Vergara-Aragon P, Gonzalez CL, Whishaw IQ. A novel skilled-reaching impairment in paw supination on the "good" side of the hemi-Parkinson rat improved with rehabilitation. J Neurosci. 2003; 23:579–586. PMID: 12533618.
Article18. Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004; 35:992–997. PMID: 14988579.
Article19. Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992; 31:100–106. PMID: 1641086.20. Han TR, Lee SU, Kim DY, Park SH, Choi DH, Park HW, et al. Influence of exercise intensity in early rehabilitation on neurological recovery after focal ischemia in rats. Korean J Stroke. 2006; 8:106–118.21. Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001; 98:4710–4715. PMID: 11296300.
Article22. Wada K, Sugimori H, Bhide PG, Moskowitz MA, Finklestein SP. Effect of basic fibroblast growth factor treatment on brain progenitor cells after permanent focal ischemia in rats. Stroke. 2003; 34:2722–2728. PMID: 14576381.
Article23. Yang YR, Wang RY, Wang PS. Early and late treadmill training after focal brain ischemia in rats. Neurosci Lett. 2003; 339:91–94. PMID: 12614902.
Article24. Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993; 11:173–189. PMID: 8338665.
Article25. Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977; 197:1092–1094. PMID: 887941.
Article26. Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002; 8:963–970. PMID: 12161747.
Article27. Snyder EY, Park KI. Limitations in brain repair. Nat Med. 2002; 8:928–930. PMID: 12205449.
Article28. Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000; 97:12846–12851. PMID: 11070094.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Continuous Epidural Electrical Stimulation on Synapse and Neuronal Cell in Rat with Focal Ischemia
- Role of the Cerebral Cortex on Vestibular Compensation Following Unilateral Labyrinthectomy in Rats
- Electrical Stimulation for the Treatment of Tinnitus
- Effect of Conditioned Ischemic Stresses on NF-kappaB Activation in Rat Skeletal Muscle
- Electrical stimulation can facilitate vestibular compensation following unilateral labyrinthectomy in rats