J Nutr Health.
2014 Apr;47(2):106-112. 10.4163/jnh.2014.47.2.106.
Protective effects of Acanthopanax koreanum Kakai extract against carbon tetrachloride-induced liver injury in Sprague-Dawley rats
- Affiliations
-
- 1Department of Nutritional Science and Food Management, Soongeui Women's College, Seoul 100-751, Korea.
- 2Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 120-750, Korea. orank@ewha.ac.kr
- KMID: 2210024
- DOI: http://doi.org/10.4163/jnh.2014.47.2.106
Abstract
- PURPOSE
This study was conducted in order to investigate the protective effects of ethanolic extract of Acanthopanax koreanum Nakai (AE) against carbon tetrachloride (CCl4)-induced liver injury in rats.
METHODS
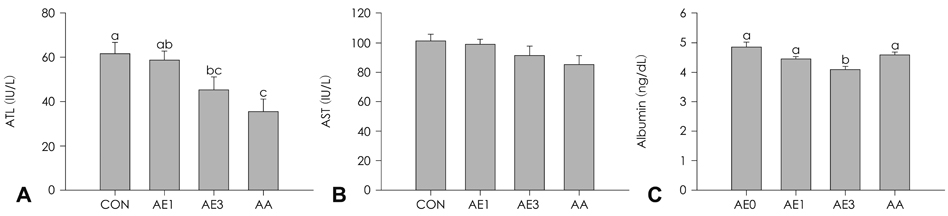
Male Sprague-Dawley rats were randomly divided into four groups in order to receive the following experimental diets with intraperitoneal injection of CCl4 (2.0 mL/kg body weight, 20% solution 0.65 mL) for eight weeks (n = 8 per group): CCl4 control (CON), CCl4 + AE 1% (AE1), CCl4 + AE 3% (AE3), or CCl4 + acanthoic acid 0.037%, which is equivalent to AE 3% (AA).
RESULTS
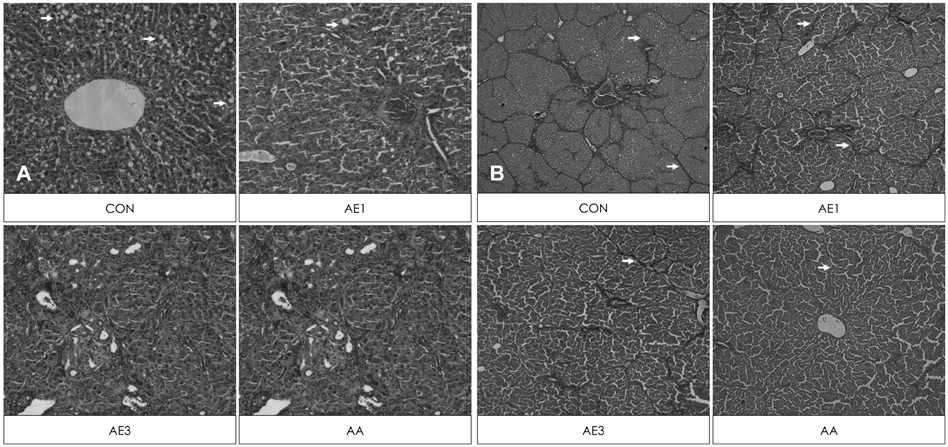
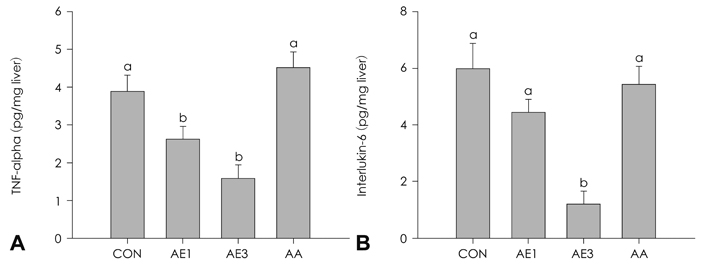
Highest serum ALT activity and albumin level were observed in the CCL4 control group, but showed a significant decrease by either AE or AA supplementation in a dose-dependent manner (p = 0.0063 and 0.0076, respectively). Both hemotoxylin and eosin staining and Masson's staining indicated remarkable prevention of CCl4-induced liver damage in the AE3 group. TNFalpha and IL-6 production were significantly lowered in the AE treated groups, but not in the AA group (p = 0.0016 and p = 0.0002, respectively). The effects of AE3 were greater than those of AA for inflammation and liver toxicity biomarkers.
CONCLUSION
Taken together, the results suggested that ethanolic extract of Acanthopanax koreanum Nakai provided hepa-toprotective effects, leading to the reduction of inflammatory response. In addition, the effect of AE was superior to that of single compound AA.
Keyword
MeSH Terms
-
Eleutherococcus*
Animals
Biomarkers
Body Weight
Carbon Tetrachloride
Carbon*
Diet
Eosine Yellowish-(YS)
Ethanol
Hematoxylin
Humans
Inflammation
Injections, Intraperitoneal
Interleukin-6
Liver*
Male
Rats
Rats, Sprague-Dawley*
Tumor Necrosis Factor-alpha
Carbon
Carbon Tetrachloride
Eosine Yellowish-(YS)
Ethanol
Hematoxylin
Interleukin-6
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Statistics Korea. Cause of death statistics [Internet]. Daejeon: Statistics Korea;2012. cited 2013 Dec 23. Available from: http://kostat.go.kr/portal/korea/kor_nw/2/6/2/index.board?bmode=read&bSeq=&aSeq=308559&pageNo=1&rowNum=10&navCount=10&currPg=&sTarget=title&sTxt=.2. Lee JH, Kim EM, Park YK, Park EJ, Sung MK, Sohn JM, Shin DY, Shin MJ, Lee HM. Pathophysiology for clinical nutrition. 1st edition. Seoul: Kyomoonsa;2013.3. Park DY, Suh KS. Experimental liver disease models of rats - morphological characteristics. Korean J Pathol. 2003; 37(3):151–158.4. McCay PB, Lai EK, Poyer JL, DuBose CM, Janzen EG. Oxygen- and carbon-centered free radical formation during carbon tetrachloride metabolism. Observation of lipid radicals in vivo and in vitro. J Biol Chem. 1984; 259(4):2135–2143.
Article5. Edwards MJ, Keller BJ, Kauffman FC, Thurman RG. The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol. 1993; 119(2):275–279.
Article6. Nan JX, Park EJ, Nam JB, Zhao YZ, Cai XF, Kim YH, Sohn DH, Lee JJ. Effect of Acanthopanax koreanum Nakai (Araliaceae) on D-galactosamine and lipopolysaccharide-induced fulminant hepatitis. J Ethnopharmacol. 2004; 92(1):71–77.
Article7. Nan JX, Jin XJ, Lian LH, Cai XF, Jiang YZ, Jin HR, Lee JJ. A diterpenoid acanthoic acid from Acanthopanax koreanum protects against D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Biol Pharm Bull. 2008; 31(4):738–742.
Article8. Jeong JE, Baek HE, Oh DS, Wi AJ, Yoon BS. Functional properties and biological activity of breeding lines, parts, and various solvents from Acanthopanax. Korean J Pharmacogn. 2013; 44(3):242–252.9. Brekhman II, Kirillov OI. Effect of eleutherococcus on alarmphase of stress. Life Sci. 1969; 8(3):113–121.
Article10. Brekhman II, Dardymov IV. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969; 9:419–430.
Article11. Ko ST, Kim SW, Lim DY. A study on the hypotensive action of Acanthopanax extract in rabbit. J Korean Pharm Sci. 1978; 8(1):6–16.12. Lim JK, Seo YB, Kim DH, Seol IC. The experimental studies on the reinforcemental effects of Acanthopanax Radicis cortex about immunity hematogenic action. Korean J Herbol. 2000; 15(1):1–17.13. Lee YS, Jung SH, Lim SS, Ji J, Lee SH, Shin KH. Effects of the water extract from the stem bark of Acanthopanax senticosus on hyperlipidemia in rats. Korean J Pharmacogn. 2001; 32(2):103–107.14. Wu YL, Jiang YZ, Jin XJ, Lian LH, Piao JY, Wan Y, Jin HR, Joon Lee J, Nan JX. Acanthoic acid, a diterpene in Acanthopanax koreanum, protects acetaminophen-induced hepatic toxicity in mice. Phytomedicine. 2010; 17(6):475–479.
Article15. Kang OH, Kim DK, Cai XF, Kim YH, Lee YM. Attenuation of experimental murine colitis by acanthoic acid from Acanthopanax koreanum. Arch Pharm Res. 2010; 33(1):87–93.
Article16. Kang HS, Kim YH, Lee CS, Lee JJ, Choi I, Pyun KH. Suppression of interleukin-1 and tumor necrosis factor-alpha production by acanthoic acid, (-)-pimara-9(11),15-dien-19-oic acid, and it antifibrotic effects in vivo. Cell Immunol. 1996; 170(2):212–221.17. Jung MG, Do GM, Shin JH, Ham YM, Park SY, Kwon O. Acanthopanax koreanum Nakai modulates the immune response by inhibiting TLR 4-dependent cytokine production in rat model of endotoxic shock. Nutr Res Pract. 2013; 7(6):460–465.18. Choi YH, Kim JW. Quantitative analysis of eleutherosides B and E using HPLC-ESI/MS. Korean J Pharmacogn. 2002; 33(2):88–91.19. Lim JH, Lee SH, Jun BS, Yang YT, Koh JS. Changes in major constituents by soaking of Acanthopanax koreanum with spirit solution. J Korean Soc Appl Biol Chem. 2005; 48(2):166–172.20. Song IH, Kim BH, Kim YK, Dong SH, Kim HJ, Lee JI, Chang YW, Chang R. Carbon tetrachloride (CCl4)-induced hepatic fibrosis in the rat. Korean J Gastroenterol. 1992; 24(6):1330–1339.21. Kim JY, Kwon O. Culinary plants and their potential impact on metabolic overload. Ann N Y Acad Sci. 2011; 1229:133–139.
Article22. Jang WJ, Kim YS, Ha YL, Park CW, Ha YK, Kim JO. Optimal level for the protection of carbon tetrachloride-induced Sprague-Dawley rat liver damage by mycelial cultures of lentinus edodes. J Life Sci. 2010; 20(5):782–788.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Protective Effects of Garlic against Carbon tetrachloride-induced Hepatotoxicity
- The Effect of Acanthopanax koreanum Extract on the Induction of Collagen Induced Arthritis for DBA/1J Mice
- Anti-oxidant activities of kiwi fruit extract on carbon tetrachloride-induced liver injury in mice
- Morphologic Change of Rat Liver Induced by Repeated Administration of Carbon Tetrachloride and Dimethylnitrosamine
- Antifibrotic activity a fermentation filtrate of Ganoderma lucidum