J Korean Diabetes Assoc.
2006 May;30(3):151-160. 10.4093/jkda.2006.30.3.151.
Protective Effect of PGC-1 on Lipid Overload-induced Apoptosis in Vascular Endothelial Cell
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, University of Ulsan College of Medicine, Korea.
- 2Asan Institute for Life Sciences, University of Ulsan College of Medicine, Korea.
- KMID: 2177606
- DOI: http://doi.org/10.4093/jkda.2006.30.3.151
Abstract
-
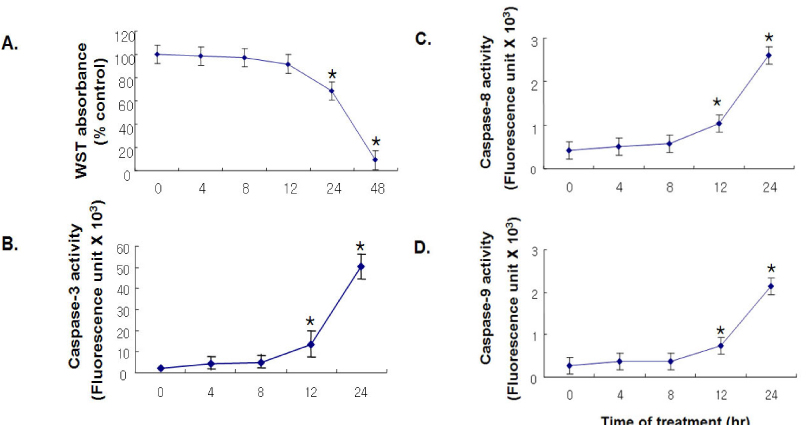
BACKGROUND: Fatty acids contribute to endothelial cell dysfunction and apoptosis by inducing accumulation of long chain fatty acyl CoA (LCAC), which increases oxidative stress in vascular endothelial cells. Forced expression of PGC-1 was shown to induce mitochondrial biogenesis and to control expression of mitochondrial enzymes involved in fatty acid oxidation. This study was undertaken to test the hypothesis that PGC-1 overexpression could prevent endothelial cell apoptosis by enhancing fatty acid oxidation and relieving oxidative stress in vascular endothelium.
METHODS
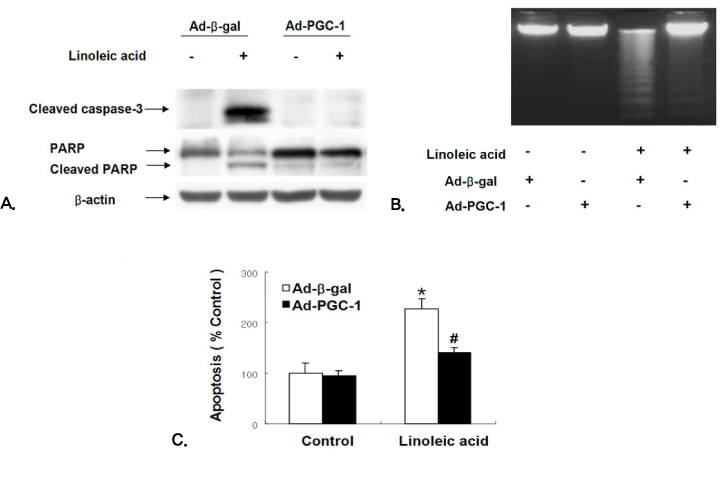
Adenoviruses containing human PGC-1 (Ad-PGC-1) and beta-galactosidase (Ad-beta-gal) were transfected to confluent human aortic endothelial cells (HAECs). To investigate the effect of adenoviral PGC-1 gene transfer on apoptosis, combined treatment of linoleic acid (LA), an unsaturated fatty acid, was performed.
RESULTS
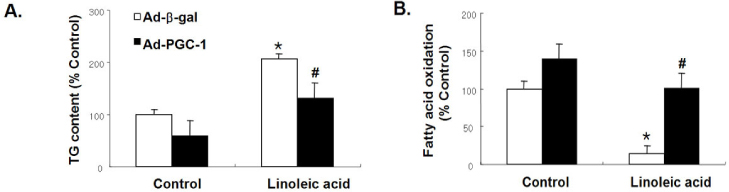
PGC-1 overexpression inhibited the increase in ROS production and apoptosis of HAECs induced by LA. Also, PGC-1 led to a significant increase in fatty acid oxidation and decrease in triglyceride content in HAECs. LA caused the decrease of adenine nucleotide translocase (ANT) activity and transient mitochondrial hyperpolarization, which was followed by depolarization. PGC-1 overexpression prevented these processes.
CONCLUSION
In summary, PGC-1 overexpression inhibited mitochondrial dysfunction and apoptosis by facilitating fatty acid oxidation and protecting against the damage from oxidative stress in HAECs. The data collectively suggest that the regulation of intracellular PGC-1 expression might play a critical role in preventing atherosclerosis.
Keyword
MeSH Terms
-
Acyl Coenzyme A
Adenoviridae
Apoptosis*
Atherosclerosis
beta-Galactosidase
Endothelial Cells*
Endothelium, Vascular
Fatty Acids
Humans
Linoleic Acid
Mitochondria
Mitochondrial ADP, ATP Translocases
Organelle Biogenesis
Oxidative Stress
Triglycerides
Acyl Coenzyme A
Fatty Acids
Linoleic Acid
Mitochondrial ADP, ATP Translocases
beta-Galactosidase
Figure
Reference
-
1. Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988. 37:1595–1607.2. Kopelman PG. Obesity as a medical problem. Nature. 2000. 404:635–643.3. Bakker SJ, Ijzerman RG, Teerlink T, Westerhoff HV, Gans RO, Heine RJ. Cytosolic triglycerides and oxidative stress in central obesity: the missing link between excessive atherosclerosis, endothelial dysfunction, and beta-cell failure? Atherosclerosis. 2000. 148:17–21.4. Hart CM, Tolson JK, Block ER. Supplemental fatty acids alter lipid peroxidation and oxidant injury in endothelial cells. Am J Physiol. 1991. 4:L481–L488.5. Kunsch C, Medford RM. Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999. 85:753–766.6. Henning B, Meerarani P, Ramadass P, Watkins BA, Toborek M. Fatty acid-mediated activation of vascular endothelial cells. Metabolism. 2000. 49:1006–1013.7. Irani K. Oxidant signalingin vascular cell growth, death, and survival: a review of the role of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000. 87:179–183.8. Egan BM, Lu G, Greene EL. Vascular effects of non-esterified fatty acids: implications for the cardiovascular risk factor cluster. Prostaglandins Leukot Essent Fatty Acids. 1999. 60:411–420.9. Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997. 100:1230–1239.10. Choy JC, Granville DJ, Hunt DW, McManus BM. Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol. 2001. 33:1673–1690.11. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked adaptive thermogenesis. Cell. 1998. 92:829–839.12. Tiraby C, Taverniner G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem. 2003. 278:33370–33376.13. Butow RA, Bahassi EM. Adaptive thermogenesis: orchestrating mitochondrial biogenesis. Curr Biol. 1999. 9:R767–R769.14. Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. Adaptation of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002. 16:1879–1886.15. Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001. 413:131–138.16. Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–256.17. Herzig S, Hedrick S, Morantte I, Koo SH, Galimi F, Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature. 2000. 426:190–193.18. Vander Heiden MG, Chandel NS, Schumacker PT, Thompson CB. Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol Cell. 1999. 3:159–167.19. Chong ZZ, Lin SH, Maiese K. Nicotinamide modulates mitochondrial membrane potential and cysteine protease activity during cerebral vascular endothelial cell injury. J Vasc Res. 2002. 39:131–147.20. Li JM, Mullen AM, Yun S, Wientjes F, Brouns GY, Thrasher AJ, Shah AM. Essential role of the NADPH oxidase subunit p47 (phox) in endothelial cell superoxide production in response to phorbol ester and tumor necrosis factor-alpha. Circ Res. 2002. 90:143–150.21. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001. 414:813–820.22. Imai H, Koumura T, Nakajima R, Nomura K, Nakagawa Y. Protection from inactivation of the adenine nucleotide translocator during hypoglycaemia-induced apoptosis by mitochondrial phospholipid hydroperoxide glutathione peroxidase. Biochem J. 2003. 371:799–809.23. Green D, Kroemer G. The central executioners of apoptosis: caspase or mitochondria? Trends Cell Biol. 1998. 8:267–271.24. Paradis E, Clavel S, Bouillaud F, Ricquier D, Richard D. Uncoupling protein 2: a novel player in neuroprotection. Trends Mol Med. 2003. 9:522–525.25. Khaled AR, Reynolds DA, Young HA, Thompson CB, Muegge K, Durum SK. Interleukin-3 withdrawal induces an early increase in mitochondrial membrane potential unrelated to the Bcl-2 family. J Biol Chem. 2001. 276:6453–6462.26. Armstrong JS, Jones DP. Glutathione depletion enforces the mitochondrial permeability transition and causes cell death in Bcl-2 overexpressing HL60 cells. FASEB J. 2002. 16:1263–1265.27. Widmann C, Gibson S, Johnson GL. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem. 1998. 273:7141–7147.28. Lee KU, Lee IK, Han J, Song DK, Kim YM, Song HS, Kim HS, Lee WJ, Koh EH, Song KH, Han SM, Kim MS, Park IS, Park JY. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005. 96:1200–1207.29. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999. 98:115–124.30. Lehman J, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelley DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000. 106:847–856.31. Louet JF, Hayhurst G, Gonzalez FJ, Girard J. The coactivator PGC-1 is involved in the regulation of the liver carnitine palmitoyltransgerase I gene expression by cAMP in combination with HFN4 alpha and cAMP-response element-binding protein (CREB). J Biol Chem. 2002. 277:37991–38000.32. Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kullkarni R, Evans RM, Olefsky J, Montminy M. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004. 10:530–534.33. Luscher TF, Barton M. Biology of the endothelium. Clin Cardiol. 1997. 20:II-3–II-10.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retraction: Protective Effect of PGC-1 on Lipid Overload-induced Apoptosis in Vascular Endothelial Cell
- Cytotoxicity of Diamide and the Protective Effect of Thioredoxin on Diamide-Induced Vasculotoxicity in Vascular Endothelial Cells
- The Antiapoptotic Effect of Vascular Endothelial Growth Factor and Angiopoietin-1 on Glomerular Endothelial Cells in Adriamycin or Cisplatin-induced Apoptosis
- The Anti-apoptotic Effect of Adrenomedullin on Vascular Endothelial Cells in Radiocontrast Media-induced Apoptosis
- Galpha12 Protects Vascular Endothelial Cells from Serum Withdrawal-Induced Apoptosis through Regulation of miR-155